Calcitonina y proto-oncogen RET
Liliana M. Bergoglio, Bioquímica Endocrinóloga, Universidad Nacional de Córdoba, Córdoba, Argentina
E-mail: liberg@uolsinectis.com.ar
Jorge H. Mestman, Médico Endocrinólogo, Universidad del Sur de California, Los Ángeles, CA, Estados Unidos
NACB: Guía de Consenso para el Diagnóstico y Seguimiento de la Enfermedad Tiroidea Fuente: Revista Argentina de Endocrinología y Metabilismo, Vol 42, N° 2, Año 2005
Mencionamos con reconocimiento los nombres de los profesionales que participaron en la revisión de la traducción del documento original sobre el cual está basada esta monografía: Claudio Aranda, Hospital Carlos C. Durand, Buenos Aires, Argentina Aldo H. Coleoni, Universidad Nacional de Córdoba, Córdoba, Argentina.N. Liliana F. de Muñoz, Hospital de Niños de la Santísima Trinidad, Córdoba, Argentina Silvia Gutiérrez, Hospital Carlos C. Durand,Buenos Aires, Argentina H. Rubén Harach, Hospital Dr. A. Oñativia, Salta, Argentina Gustavo C. Maccallini, Hospital Carlos C. Durand, Buenos Aires, Argentina Mirta B. Miras, Hospital de Niños de la Santísima Trinidad, Córdoba, Argentina Hugo Niepomniszcze, Universidad Nacional de Buenos Aires, Buenos Aires, Argentina Adriana Oneto, Hospital Carlos C. Durand, Buenos Aires, Argentina.Eduardo Pusiol, Universidad Nacional de Cuyo Mendoza, Argentina. Gerardo C. Sartorio, Hospital J. M. Ramos Mejía, Buenos Aires, Argentina
El carcinoma medular de tiroides (CMT) se produce por una transformación maligna de las células C parafoliculares tiroideas y representa aproximadamente entre el 5 y el 8% de todos los casos de cáncer de tiroides. Aproximadamente el 75% es de presentación esporádica, en tanto el 25% restante es hereditario (9, 11, 347).
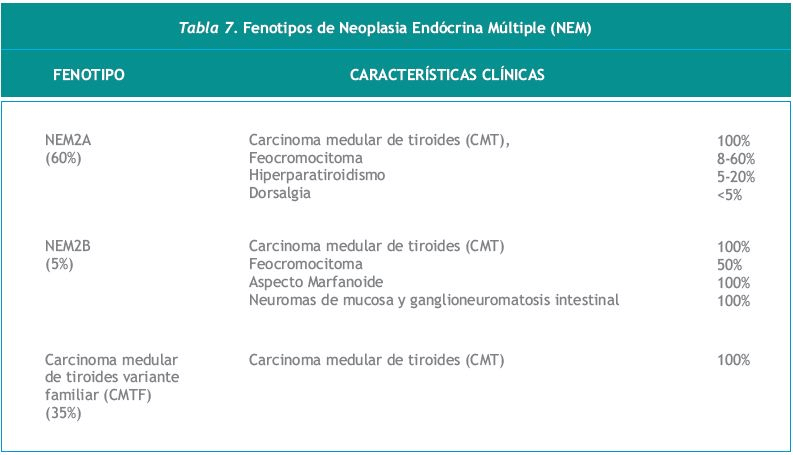
Según un estudio de patología nodular la prevalencia de CMT es de 0,57% (348). El comportamiento y el manejo del CMT medular difiere del que se observa en el carcinoma de tiroides bien diferenciado de origen folicular (346). Las formas hereditarias de CMT se presentan asociadas a síndromes poliglandulares denominados neoplasias endócrinas múltiples (NEM) tipos 2A y 2B que son, heredados de manera autosómica dominante, con penetrancia asociada a la edad, y expresión variable.
Existe la denominada variante familiar del CMT (CMTF), que se caracteriza por la aparición de CMT sin endocrinopatía asociada. En 1993 se describieron mutaciones responsables de estos trastornos en el proto-oncogen RET (349, 350), que se localiza en el cromosoma 10 sub-banda 10q11.2. Las expresiones fenotípicas de la NEM hereditaria se resumen en la Tabla 7.
1. Detección de CMT mediante la determinación de Calcitonina Sérica (CT)
(a) Biosíntesis de Calcitonina
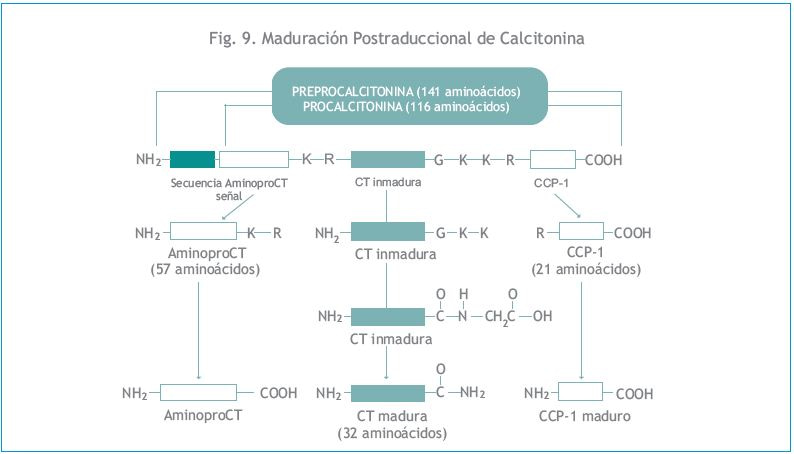
El gen CALC-1 que codifica para la CT humana se ubica en el extremo del brazo corto del cromosoma 11 (11p15.3-15.5). Si bien las células C parafoliculares tiroideas son la fuente principal de CT circulante, muchas otras categorías de células neuroendócrinas, normalmente contienen y segregan CT. La calcitonina madura es un polipéptido de 32 aminoácidos (aa) con un puente disulfuro y una amida prolínica carboxiterminal que juega un rol funcional importante. Como se muestra en la Figura 9, la CT madura es el resultado de una modificación postraduccional de un precursor de más de 141 aa (preprocalcitonina) dentro de las células C parafoliculares. La preprocalcitonina primero sufre el clivaje de su péptido señal para formar procalcitonina (proCT), una prohormona que consiste en 116 residuos de aa.
En el extremo aminoterminal de proCT hay un péptido de 57 aa, denominado aminoprocalcitonina (aminoproCT o PAS-57), y en el extremo carboxiterminal, un péptido de 21 aa conocido como péptido-1 carboxiterminal de calcitonina (CCP-1 o Katacalcina). Los 33 aa de la porción central de la molécula de proCT constituyen la molécula de CT inmadura. La CT madura activa de 32 aminoácidos (que incluye una prolina amidada en su extremo carboxiterminal) se produce a partir de CT inmadura por acción de la enzima monoxidasa amidante de peptidilglicina (PAM).
(b) Métodos de determinación de CT
Hasta 1988, los métodos de ensayo para la determinación de CT se basaban principalmente en el radioinmunoensayo y utilizaban anticuerpos policlonales que reconocían tanto el monómero de CT madura como otras formas circulantes (precursores y productos de degradación).
Estos primeros ensayos carecían de especificidad y sensibilidad. Desde 1988, las mejoras con las nuevas técnicas inmunométricas basadas en el uso de anticuerpos monoclonales (uno capaz de identificar la región N-terminal y el otro, la región C-terminal) han permitido desarrollar ensayos más específicos y sensibles para la CT madura monomérica de 32 aa. Actualmente los ensayos inmunométricos de dos sitios detectan CT en plasma en ayunas en el 83% y 46% de hombres y mujeres sanos, respectivamente (351-353). Los valores de CT pueden diferir según el método utilizado, lo que dificulta la interpretación de los resultados. Es importante que los médicos conozcan que las diferencias entre métodos existen y pueden afectar la interpretación y el uso adecuado de la CT en el diagnóstico y el manejo del CMT.
(c) Valores Basales de Calcitonina
En 1968 se estableció que los valores basales de calcitonina eran un marcador útil para el diagnóstico de CMT (354). En la actualidad los IMA de dos sitios, específicos para CT madura, típicamente informan niveles de CT por debajo de 10 ng/L (pg/ml) para los controles normales sanos y para el 90% de los pacientes con otra disfunción tiroidea que no sea CMT. (348, 355- 357).
| Recomendación Nº 51. Ensayos para CT |
| *La CT madura (de 32 aminoácidos) es el principal marcador tumoral en el CMT.*Las determinaciones de CT aplicadas al diagnóstico y seguimiento del CMT deberían realizarse mediante ensayos inmunométricos de dos sitios, específicos para el monómero maduro de CT de 32 aminoácidos.*Actualmente, los valores basales de CT inferiores a 10 pg/ml (ng/L) son considerados como normales.A medida que se disponga de nuevos ensayos más sensibles, dicho umbral debería redefinirse. |
Los pacientes con formas micro o macro de CMT (variantes esporádicas o familiares) poseen valores elevados de CT que correlacionan con la masa tumoral (358). La hiperplasia de células C (HCC) es el hallazgo histológico más temprano, previo al desarrollo de un microcarcinoma, en los pacientes con NEM2. La HCC se presenta pronto luego del nacimiento, y en esta etapa de la enfermedad la CT basal puede ser normal.
Por lo tanto un resultado basal normal de CT no descarta patología de células C en las etapas más tempranas.
(d) Pruebas de Estimulación de Calcitonina para el Diagnóstico de CMT
Para detectar de manera temprana las anormalidades en las células C, se han usado pruebas de estimulación con secretagogos conocidos de la CT como el calcio y un análogo de la gastrina (pentagastrina, Pg y cuando la Pg no está disponible, el omeprazol) ya sea en forma separada o combinada, que provocan un aumento en la CT en todos los estadios del CMT (359-364). Una ventaja de estas pruebas es que pueden detectar hiperplasia de células C antes de confirmarse el CMT. En los países, en los que la utilización de técnicas de genética molecular es accesible, la cirugía para los portadores se basa exclusivamente en la prueba genética, y las pruebas de estimulación se usan raramente. Lo mismo sucede en países en donde la pentagastrina es difícil de obtener.
| Recomendación Nº 52. Utilidad Clínica de la determinación de CT para Diagnóstico de CMT |
| *Los ensayos de CT son método-dependientes lo cual puede tener un impacto en la interpretación de losresultados de CT.*En pacientes con enfermedades tiroideas autoinmunes (tiroiditis de Hashimoto o enfermedad de Graves) pueden observarse valores elevados de CT.*El primer hallazgo histológico previo al desarrollo de un microcarcinoma es la hiperplasia de células C (HCC), la cual puede no estar acompañada de una CT elevada en los primeros estadios de un CMT.*Un aumento en los valores basales de CT por encima de 10 pg/ml (ng/L) sugiere un CMT en la etapa de microcarcinoma.*Generalmente existe una correlación positiva entre valores de CT y masa tumoral. |
Las pruebas de estimulación se usan habitualmente:
*Para confirmar el diagnóstico de CMT antes de la cirugía cuando los niveles basales de CT están sólo moderadamente elevados (menos de 100 pg/ml).
*Para detectar enfermedad de células C en portadores RET positivos.
*Para el control prequirúrgico de niños RET positivos.
*Para el control postoperatorio de recurrencia de tumores.
*Cuando la prueba genética no está fácilmente disponible.
(i) Prueba de Estimulación con Pentagastrina
La prueba de estimulación con Pg se ha utilizado ampliamente en el diagnóstico de CMT pero en
muchos países no es muy accesible (359, 365). La misma consiste en una infusión endovenosa
de Pg (0,5 ug/kg/peso corporal) efectuada durante unos 5 segundos. Esta administración “lenta” de Pg reduce los efectos secundarios transitorios (náuseas, vómitos, compresión subesternal, rubor, y hormigueo en las extremidades) y mejora la tolerancia del paciente a la prueba. Se toman muestras basales, y 1, 2, 5, y a veces 10 minutos después de iniciada la infusión.
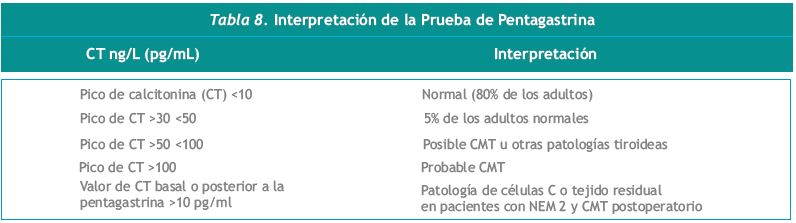
La Tabla 8 muestra los resultados y la interpretación de los valores de CT estimulada con Pg. El pico de estimulación normalmente es inferior a 10 ng/L (pg/ml) en el 80% de adultos voluntarios sanos, e inferior a 30 ng/L (pg/ml) en el 95% de la población general. Los varones normales tienen valores más altos que las mujeres. Una prueba positiva [pico de CT superior a 100 ng/L (pg/ml)] sugiere CMT. En los pacientes que tienen la mutación familiar responsable de la NEM2, un pico entre 30 y 100 ng/L (pg/ml) es típicamente revelador de una HCC o de un microcarcionoma. Aunque se ha observado que un pico de CT inferior a 100 ng/L (pg/ml) puede darse en adultos con otras enfermedades tiroideas que no sean CMT (ver la Tabla 9), nunca se han observado tales resultados en niños menores de 12 años que no sean portadores de mutación RET (366). La ausencia de CT elevada en individuos jóvenes con mutación del RET no excluye la posibilidad de que el CMT se desarrolle posteriormente.
No se ha establecido la mejor edad para realizar la prueba de Pg en niños portadores de la mutación del RET para NEM2 ya que esta varía con el tipo de mutación y el tipo de NEM2 presentes en sus familias (367, 368). Por lo tanto, los portadores de la mutación con valores basales normales de CT deberían someterse a pruebas genéticas o de estimulación lo más pronto posible después del nacimiento para NEM2B, y a los 2 años de edad para NEM2. Sin embargo, debería destacarse que normalmente se observan valores altos de CT en neonatos, seguidos de un descenso asociado a la edad, desde el nacimiento hasta el año, y aún no se dispone de datos sobre pruebas de estimulación para este grupo de edad (369).
Esta prueba debería repetirse una vez al año como mínimo, hasta que de positiva, momento en el cual debería realizarse una tiroidectomía total. Pero dado el pronóstico del CMT, la baja tolerancia a la prueba con Pg, y las repercusiones psicológicas para la familia, algunos médicos prefieren no seguir este procedimiento y optan (como se prefiere actualmente) por realizar una tiroidectomía a todos los portadores de la mutación del RET entre los 4 y 5 años de edad.
(ii) Prueba de Estimulación con Calcio
Esta prueba consiste en administrar por vía endovenosa, durante 30 segundos, 2,5 mg/kg de gluconato de calcio. Se toman muestras para CT basal, y a 1, 2 y 5 minutos después de la inyección. Se sospecha hiperplasia de células C si la CT es mayor a 100 ng/L. En esta prueba no se han observado efectos adversos importantes, a excepción de una moderada y transitoria sensación de calor generalizado. Se ha informado que la prueba de estimulación con calcio es menos sensible que la de Pg para el diagnóstico de CMT (370-372). Además, esta prueba no ha sido evaluada usando un ensayo inmunométrico específico para el monómero maduro de CT y, por lo tanto, debe ser reevaluada con los ensayos actuales. Se ha demostrado que la prueba de estimulación con Calcio combinado con Pg potencia la sensibilidad de la prueba con Pg sola (359), resultando así en el ensayo más sensible para medir la existencia de tejido de células C.
(e) CT Basal y Post Estimulación en el Seguimiento de Pacientes después de la cirugía
Luego de la tiroidectomía, la CT sérica es el marcador tumoral aceptado para la detección de tejido tiroideo residual o de metástasis. Un valor de CT detectable, basal o post estimulación, indica la presencia de tejido tumoral (373, 374).
| Recomendación Nº 53. Seguimiento Postoperatorio del CMT |
| *La CT y el CEA deberían determinarse inmediatamente antes y 6 meses después de la cirugía del CMT. En algunos pacientes los niveles de CT disminuyen lentamente. La primera determinación de CT postoperatoria no debería realizarse antes de las 2 semanas.*La presencia de tejido residual o la recurrencia del CMT sólo pueden descartarse si ambos niveles de CT, basal y post estímulo son indetectables. |
Teniendo en cuenta las variaciones en la velocidad de desaparición de la CT sérica, la primera muestra control postoperatoria no debería tomarse, antes de las 2 semanas después de la cirugía (375). Cabe destacar que el antígeno carcinoembrionario (CEA) que se determina junto con la CT para detectar recurrencia, parece ser un marcador útil de desdiferenciación en el CMT y es indicativo de pobre pronóstico en el seguimiento.
(f) Niveles Elevados de Calcitonina en otras patologías además de CMT
Como se muestra en la Tabla 9, se han observado niveles elevados de calcitonina en otras patologías, además del CMT y de los tumores neuroendócrinos. En enfermedades tiroideas autoinmunes (tiroiditis de Hashimoto y enfermedad de Graves) se suele observar una mayor liberación de CT (376-378). Entre las enfermedades no tiroideas con elevado nivel de CT se incluyen insuficiencia renal severa, hipercalcemia e hipergastrinemia, enfermedades inflamatorias agudas de pulmón y otras formas locales o generales de sepsis (enfermedad de Biermer, t ras tornos iatrogénicos, etc.) (379-381).
Como en algunos casos los niveles elevados de CT fueron detectados por RIA policlonal, estos informes requieren confirmación con los ensayos actuales basados en anticuerpos monoclonales que son más específicos para CT madura. Estudios que utilizaron un antisuero específico contra ProCT, CT y CCP-1, junto con HPLC y filtración con gel, demostraron que los pacientes con un elevado nivel de CT asociado a enfermedad no tiroidea mostraban un notable aumento en sus niveles séricos de ProCT intacta y, en menor grado, de la forma no escindida, CTCCP-1. Por lo general, dichos pacientes presentan niveles normales o ligeramente elevados de CT madura. Usando antisuero específico de epitopes y técnicas de aislamiento se ha podido demostrar que otros tumores que no son CMT pueden segregar grandes cantidades de CT madura y diversos precursores (382).Esto puede observarse en varios tumores neuroendócrinos, especialmente en el cáncer de pulmón de células pequeñas y en el carcinoide bronquial. Sin embargo, en estos pacientes, se observa sólo un ligero incremento, o ninguno, en los niveles de CT, después de la prueba de estimulación con Pg. (383). La hiperplasia de células C se presenta en la tiroiditis linfocítica y en algunos pacientes con cáncer diferenciado de tiroides (384-386). Esta HCC puede ser responsable de valores ligeramente elevados de CT madura y de la respuesta aumentada de CT con la prueba de estimulación combinada o con de PG solamente.

2. Detección de Cáncer Medular de Tiroides mediante la determinación de mutaciones en el Proto-oncogen RET
Hasta 1987 el único método disponible para detectar sujetos de riesgo de CMT era realizar repetidas determinaciones de CT estimulada en el grupo familiar de los pacientes afectados. La subsiguiente identificación del locus 10q11.2 responsable de la NEM2 en el cromosoma 10 hizo posible la detección de sujetos portadores a través del screening genético (378). Se ha establecido que diversos tipos de mutaciones en el cromosoma 10 pueden activar el Protooncogen RET responsable de la NEM2 (349, 350). Esto permite realizar un estudio sistemático del problema antes de que aparezcan los primeros signos biológicos. Actualmente, en muchos países desarrollados, los estudios genéticos
constituyen la primera estrategia diagnóstica. Sin embargo, para una predicción efectiva de la enfermedad, es necesario que los resultados positivos del screening genético se complementen con un exhaustivo estudio de los miembros sanos y enfermos de la familia.
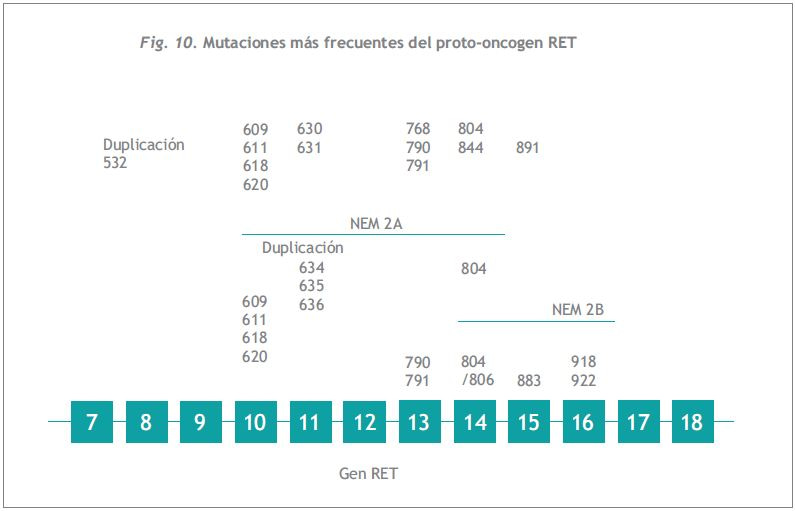
El RET es un gen de 21 exones que codifica para un receptor de membrana del tipo tirosina quinasa. Este receptor se caracteriza por una región cadherina simil en el dominio extracelular, una región rica en cisteína inmediatamente externa a la membrana y un dominio intracelular de tirosina quinasa. Como se muestra en la Figura 10, las mutaciones descriptas hasta ahora en la NEM2 se hallan en los exones 8, 10, 11, 13, 14, 15 y 16 (368, 387-391).
(a) Screening Genético para el Diagnóstico de NEM2
La NEM2 es una enfermedad familiar autosómica dominante, causada por la activación de mutaciones en el proto-oncogen RET (349). Aproximadamente un 75% de todos los CMT son de origen esporádico y único. El 44% de dichos tumores presenta una mutación somática en el codón 918 (392). Se les debe realizar screening a todos los miembros colaterales de la familia, ancestros y descendientes del caso índice, así como a todos los descendientes de los miembros afectados. El sceening se basa en la identificación de la mutación genómica del proto-oncogen RET usando análisis de secuencia de DNA genómico del caso índice y en la búsqueda sistemática de esta mutación en todos los miembros de la familia potencialmente afectados (Figura 10) (393, 394).
Las mutaciones responsables de la variedad NEM2A afectan principalmente al dominio extracelular rico en cisteína, resultando que cada una de estas mutaciones transforma una cisteína en otro aminoácido. Las principales mutaciones encontradas se ubican en los codones 609, 611, 618 y 620 del exón 10 y en el codón 634 del exon 11(368, 378). El carcinoma medular de tiroides familiar (CMTF) está generalmente asociado a mutaciones en los codones descriptos del exón 10, así como en los codones 768 y 804 de los exones 13 y 14 (368).
La mayoría (87%) de las mutaciones en el codón 634 del exón 11 están asociadas a las manifestaciones en múltiples órganos de NEM2A (CMT, feocromocitoma e hiperparatiroidismo) (9, 378).
Los tumores asociados a NEM2B son causados por mutaciones en el dominio intracelular de tirosina quinasa 2 (TK2). La mayoría (97%) de los casos de NEM 2B involucran al codón 918 en el exón 16, que provoca el cambio de una metionina por treonina, la cual se presenta con frecuencia en forma de nuevas mutaciones (“de novo”) de la línea germinal (395). El menor porcentaje (5%) de mutaciones de NEM2B afecta al codón 883 del exón 15 ó 922 del exón 16 (378, 394). Una correlación entre fenotipo y genotipo sugiere que en pacientes afectados por CMTF con mutaciones del RET que no afectan a las cisteínas, la enfermedad de células C aparece más tardíamente que en aquellos pacientes que padecen las mutaciones del RET clásicas del exón 10 (368, 396).
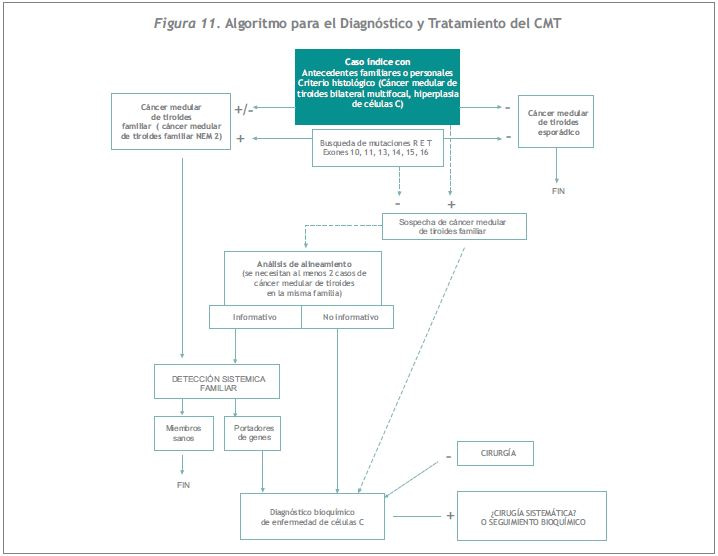
Una vez identificada una mutación en una familia, se puede tener la certeza de que los miembros de dicha familia y sus descendientes que no presentan esa mutación se encuentran libres de la patología. Por el contrario, los sujetos portadores de la mutación padecen la patología y requerirán tratamiento quirúrgico para manejar o prevenir el desarrollo de la enfermedad (Figura 11). Si se identifica una mutación no genómica en el caso índice, como sucede en menos del 3% de las NEM2A y en el 5% de los CMTF, se puede predecir el nivel de riesgo
para los miembros de la familia mediante el análisis de ligadura. Si la genealogía familiar no permite efectuar predicciones de este tipo, la enfermedad deberá detectarse repitiendo estudios clínicos y pruebas biológicas específicas a intervalos apropiados.
| Recomendación Nº 54. Riesgo Genético de Carcinoma Medular de Tiroides |
| *En NEM2, el porcentaje de miembros de la familia potencialmente afectados por la enfermedad es del50%.*Casi todos los pacientes portadores de mutaciones del RET desarrollarán CMT. (Nota: las mutaciones inactivantes del gen RET también causan la enfermedad de Hirschsprung.)*Se encontró que el 5-10% de los CMT presenta mutaciones del RET de la línea germinal. Por lo tanto, el análisis del RET se justifica en todos los pacientes con CMT aparentemente esporádico. |
Referencias Bibliográficas
1. Nohr SB, Laurberg P, Borlum KG, Pedersen Km, Johannesen PL, Damm P. Iodine deficiency in pregnancy in Denmark. Regional variations and frequency of individual iodine supplementation. Acta Obstet Gynecol Scand 1993;72:350-3.
2. Glinoer D. Pregnancy and iodine. Thyroid 2001;11:471-81.
3. Hollowell JG, Staehling NW, Hannon WH, Flanders DW, Gunter EW, Maberly GF et al. Iodine nutrition in the Unites States. Trends and public health implications: iodine excretion data from National Health and Nutrition Examination Surveys I and III (1971-1974 and 1988-1994). J Clin Endocrinol Metab 1998;83:3398-400.
4. Wartofsky L, Glinoer D, Solomon d, Nagataki S, Lagasse R, Nagayama Y et al. Differences and similarities in the diagnosis and treatment of Graves disease in Europe, Japan and the United States. Thyroid 1990;1:129-35.
5. Singer PA, Cooper DS, Levy EG, Ladenson PW, Braverman LE, Daniels G et al. Treatment guidelines for patients with hyperthyroidism and hypothyroidism. JAMA 1995;273:808-12.
6. Singer PA, Cooper DS, Daniels GH, Ladenson PW, Greenspan FS, Levy EG et al. Treatment Guidelines for Patients with Thyroid Nodules and Well-differentiated Thyroid Cancer. Arch Intern Med 1996;156:2165-72.
7. Vanderpump MPJ, Ahlquist JAO, Franklyn JA and Clayton RN. Consensus statement for good practice and audit measures in the management of hypothyroidism and hyperthyroidism. Br Med J 1996;313:539-44.
8. Laurberg P, Nygaard B, Glinoer D, Grussendorf M and Orgiazzi J. Guidelines for TSH-receptor antibody measurements in pregnancy: results of an evidence-based symposium organized by the European Thyroid Association. Eur J Endocrinol 1998;139:584-6.
9. Cobin RH, Gharib H, Bergman DA, Clark OH, Cooper DS, Daniels GH et al. AACE/AAES Medical/Surgical Guidelines for Clinical Practice: Management of Thyroid Carcinoma. Endocrine Pract 2001;7:203-20.
10. Ladenson PW, Singer PA, Ain KB, Bagchi N, Bigos ST, Levy EG et al. American Thyroid Association Guidelines for detection of thyroid dysfunction. Arch Intern Med 2000;160:1573-5.
11. Brandi ML, Gagel RJ, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C et al. Consensus Guidelines for Diagnosis and Therapy of MEN Type 1 and Type 2. J Clin Endocrinol Metab 2001;86:5658-71.
12. Werner and Ingbar’s “The Thyroid”. A Fundamental and Clinical Text. Lippincott-Raven, Philadelphia 2000. Braverman LE and Utiger RD eds.
13. DeGroot LJ, Larsen PR, Hennemann G, eds. The Thyroid and Its Diseases. (www.thyroidmanager.org) 2000.
14. Piketty ML, D’Herbomez M, Le Guillouzic D, Lebtahi R, Cosson E, Dumont A et al. Clinical comparison of three labeled-antibody immunoassays of free triiodothyronine. Clin Chem 1996;42:933-41.
15. Sapin R, Schlienger JL, Goichot B, Gasser F and Grucker D. Evaluation of the Elecsys free triiodothyronine assay; relevance of age-related reference ranges. Clin Biochem 1998;31:399-404.
16. Robbins J. Thyroid hormone transport proteins and the physiology of hormone binding. In “Hormones in Blood”. Academic Press, London 1996. Gray CH, James VHT, eds. pp 96-110.
17. Demers LM. Thyroid function testing and automation. J Clin Ligand Assay 1999;22:38-41.
18. Hollowell JG, Staehling NW, Hannon WH, Flanders WD, Gunter EW, Spencer CA et al. Serum thyrotropin, thyroxine and thyroid antibodies in the United States population (1988 to 1994): NHANES III. J Clin Endocrinol Metab 2002;87:489-99.
19. Wardle CA, Fraser WD and Squire CR. Pitfalls in the use of thyrotropin concentration as a first-line thyroid-function test. Lancet 2001;357:1013-4.
20. Spencer CA, LoPresti JS, Patel A, Guttler RB, Eigen A, Shen D et al. Applications of a new chemiluminometric thyrotropin assay to subnormal measurement. J Clin Endocrinol Metab 1990;70:453-60.
21. Meikle, A. W., J. D. Stringham, M. G. Woodward and J. C. Nelson. Hereditary and environmental influences on the variation of thyroid hormones in normal male twins. J Clin Endocrinol Metab1 1988;66:588-92.
22. Andersen S, Pedersen KM, Bruun NH and Laurberg P. Narrow individual variations in serum T4 and T3 in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab 2002;87:1068-72.
23. Cooper, D. S., R. Halpern, L. C. Wood, A. A. Levin and E. V. Ridgway. L-thyroxine therapy in subclinical hypothyroidism. Ann Intern Med 1984;101:18-24.
24. Biondi B, Fazio E, Palmieri EA, Carella C, Panza N, Cittadini A et al. Left ventricular diastolic dysfunction in patients with subclinical hypothyroidism. J Clin Endocrinol Metab 1999;2064-7.
25. Hak AE, Pols HAP, Visser TJ, Drexhage HA, Hofman A and Witteman JCM. Subclinical Hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med 2000;132:270-8.
26. Michalopoulou G, Alevizaki M, Piperingos G, Mitsibounas D, Mantzos E, Adamopoulos P et al. High serum cholesterol levels in persons with ‘high-normal’ TSH levels: should one extend the definition of subclinical hypothyroidism? Eur J Endocrinol 1998;138:141-5.
27. Beck-Peccoz P, Brucker-Davis F, Persani L, Smallridge RC and Weintraub BD. Thyrotropin-secreting pituitary tumors. Endocrine Rev 1996;17:610-38.
28. Brucker-Davis F, Oldfield EH, Skarulis MC, Doppman JL and Weintraub BD. Thyrotropin-secreting pituitary tumors: diagnostic criteria, thyroid hormone sensitivity and treatment outcome in 25 patients followed at the National Institutes of Health. J Clin Endocrinol Metab 76 1999;:1089-94.
29. Oliveira JH, Persani L, Beck-Peccoz P and Abucham J. Investigating the paradox of hypothyroidism and increased serum thyrotropin (TSH) levels in Sheehan’s syndrome: characterization of TSH carbohydrate content and bioactivity. J Clin Endocrinol Metab 2001;86:1694-9.
30. Uy H, Reasner CA and Samuels MH. Pattern of recovery of the hypothalamic-pituitary thyroid axis following radioactive iodine therapy in patients with Graves’ disease. Amer J Med 1995;99:173-9.
31. Hershman JM, Pekary AE, Berg L, Solomon DH and Sawin CT. Serum thyrotropin and thyroid hormone levels in elderly and middle-aged euthyroid persons. J Am Geriatr Soc 1993;41:823-8.
32. Fraser CG. Age-related changes in laboratory test results. Clinical applications. Drugs Aging 1993;3:246-57.
33. Fraser CG. 2001. Biological Variation: from principles to practice. AACC Press, Washington DC.
34. Drinka PJ, Siebers M and Voeks SK. Poor positive predictive value of low sensitive thyrotropin assay levels for hyperthyroidism in nursing home residents. South Med J 1993;86:1004-7.
35. Vanderpump MPJ, Tunbridge WMG, French JM, Appleton D, Bates D, Rodgers H et al. The incidence of thyroid disorders in the community; a twenty year follow up of the Whickham survey. Clin Endocrinol 1995;43:55-68.
36. Sawin CT, Geller A, Kaplan MM, Bacharach P, Wilson PW, Hershman JM et al. Low serum thyrotropin (thyroid stimulating hormone) in older persons without hyperthyroidism. Arch Intern Med 1991;151:165-8.
37. Parle JV, Maisonneuve P, Sheppard MC, Boyle P and Franklyn JA. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year study. Lancet 2001;358:861-5.
38. Nelson JC, Clark SJ, Borut DL, Tomei RT and Carlton EI. Age-related changes in serum free thyroxine during childhood and adolescence. J Pediatr 1993;123:899-905.
39. Adams LM, Emery JR, Clark SJ, Carlton EI and Nelson JC. Reference ranges for newer thyroid function tests in premature infants. J Pediatr 1995;126:122-7.
40. Lu FL, Yau KI, Tsai KS, Tang JR, Tsao PN and Tsai WY. Longitudinal study of serum free thyroxine and thyrotropin levels by chemiluminescent immunoassay during infancy. T’aiwan Erh K’o i Hseh Hui Tsa Chih 1999;40:255-7.
41. Zurakowski D, Di Canzio J and Majzoub JA. Pediatric reference intervals for serum thyroxine, triiodothyronine, thyrotropin and free thyroxine. Clin Chem 1999;45:1087-91.
42. Fisher DA, Nelson JC, Carlton Ei and Wilcox RB. Maturation of human hypothalamic-pituitary-thyroid function and control. Thyroid 2000;10:229-34.
43. Fisher DA, Schoen EJ, La Franchi S, Mandel SH, Nelson JC, Carlton EI and Goshi JH. The hypothalamic-pituitary-thyroid negative feedback control axis in children with treated congenital hypothyroidism. J Clin Endocrinol Metab 2000;85:2722-7.
44. Penny R, Spencer CA, Frasier SD and Nicoloff JT. Thyroid stimulating hormone (TSH) and thyroglobulin (Tg) levels decrease with chronological age in children and adolescents. J Clin Endocrinol Metab 1983;56:177-80.
45. Verheecke P. Free triiodothyronine concentration in serum of 1050 euthyroid children is inversely related to their age. Clin Chem 1997;43:963-7.
46. Glinoer D, De Nayer P, Bourdoux P, Lemone M, Robyn C, van Steirteghem A et al. Regulation of maternal thyroid function during pregnancy. J Clin Endocrinol Metab 1990;71:276-87.
47. Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocrinol Rev 1997;18:404-33.
48. Weeke J, Dybkjaer L, Granlie K, Eskjaer Jensen S, Kjaerulff E, Laurberg P et al. A longitudinal study of serum TSH and total and free iodothyronines during normal pregnancy. Acta Endocrinol 1982;101:531-7.
49. Pedersen KM, Laurberg P, Iversen E, Knudsen PR, Gregersen HE, Rasmussen OS et al. Amelioration of some pregnancy associated variation in thyroid function by iodine supplementation. J Clin Endocrinol Metab 1993;77:1078-83.
50. Nohr SB, Jorgensen A, Pedersen KM and Laurberg P. Postpartum thyroid dysfunction in pregnant thyroid peroxidase antibody-positive women living in an area with mild to moderate iodine deficiency: Is iodine supplementation safe? J Clin Endocrinol Metab 2000;85:3191-8.
51. Panesar NS, Li CY and Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem 2001;38:329-32.
52. Nissim M, Giorda G, Ballabio M, D’Alberton A, Bochicchio D, Orefice R et al. Maternal thyroid function in early and late pregnancy. Horm Res 1991;36:196-202.
53. Talbot JA, Lambert A, Anobile CJ, McLoughlin JD, Price A, Weetman AP et al. The nature of human chorionic gonadotrophin glycoforms in gestational thyrotoxicosis. Clin Endocrinol 2001;55:33-9.
54. Jordan V, Grebe SK, Cooke RR, Ford HC, Larsen PD, Stone PR et al. Acidic isoforms of chorionic gonadotrophin in European and Samoan women are associated with hyperemesis gravidarum and may be thyrotrophic. Clin Endocrinol 1999;50:619-27.
55. Goodwin TM, Montoro M, Mestman JH, Pekary AE and Hershman JM. The role of chorionic gonadotropin in transient hyperthyroidism of hyperemesis gravidarum. J Clin Endocrinol Metab 1992;75:1333-7.
56. Hershman JM. Human chorionic gonadotropin and the thyroid: hyperemesis gravidarum and trophoblastic tumors. Thyroid 1999;9:653-7.
57. McElduff A. Measurement of free thyroxine (T4) in pregnancy. Aust NZ J Obst Gynecol 1999;39:158-61.
58. Christofides, N., Wilkinson E, Stoddart M, Ray DC and Beckett GJ. Assessment of serum thyroxine binding capacity-dependent biases in free thyroxine assays. Clin Chem 1999;45:520-5.
59. Roti E, Gardini E, Minelli R, Bianconi L, Flisi M,. Thyroid function evaluation by different commercially available free thyroid hormone measurement kits in term pregnant women and their newborns. J Endocrinol Invest 1991;14:1-9.
60. Stockigt JR. Free thyroid hormone measurement: a critical appraisal. Endocrinol Metab Clin N Am 2001;30:265-89.
61. Mandel SJ, Larsen PR, Seely EW and Brent GA. Increased need for thyroxine during pregnancy in women with primary hypothyroidism. NEJM 1990;323:91-6.
62. Burrow GN, Fisher DA and Larsen PR. Maternal and fetal thyroid function. N Engl J Med 1994;331:1072-8.
63. Pop VJ, De Vries E, Van Baar AL, Waelkens JJ, De Rooy HA, Horsten M et al. Maternal thyroid peroxidase antibodies during pregnancy: a marker of impaired child development? J Clin Endocrinol Metab 1995;80:3561-6.
64. Haddow JE, Palomaki GE, Allan WC, K. G. Williams JR, Gagnon J, O’Heir CE et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. NEJM 1999;341:549-55.
65. Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol 1999;50:147-8.
66. Radetti G, Gentili L, Paganini C, Oberhofer R, Deluggi I and Delucca A. Psychomotor and audiological assessment of infants born to mothers with subclinical thyroid dysfunction in early pregnancy. Minerva Pediatr 2000;52:691-8.
67. Surks MI and Sievert R. Drugs and thyroid function. NEJM 1995;333:1688-94.
68. Kailajarvi M, Takala T, Gronroos P, Tryding N, Viikari J, Irjala K et al. Reminders of drug effects on laboratory test results. Clin Chem 2000;46:1395-1400.
69. Brabant A, Brabant G, Schuermeyer T, Ranft U, Schmidt FW, Hesch RD et al. The role of glucocorticoids in the regulation of thyrotropin. Acta Endocrinol 1989;121:95-100.
70. Samuels MH and McDaniel PA. Thyrotropin levels during hydrocortisone infusions that mimic fasting-induced cortisol elevations: a clinical research center study. J Clin Endocrinol Metab 1997;82:3700-4.
71. Kaptein EM, Spencer CA, Kamiel MB and Nicoloff JT. Prolonged dopamine administration and thyroid hormone economy in normal and critically ill subjects. J Clin Endocrinol Metab 1980;51:387-93.
72. Geffner DL and Hershman JM. Beta-adrenergic blockade for the treatment of hyperthyroidism. Am J Med 1992;93:61-8.
73. Meurisse M, Gollogly MM, Degauque C, Fumal I, Defechereux T and Hamoir E. Iatrogenic thyrotoxicosis: causal circumstances, pathophysiology and principles of treatment- review of the literature. World J Surg 2000;24:1377-85.
74. Martino E, Aghini-Lombardi F, Mariotti S, Bartelena L, Braverman LE and Pinchera A. Amiodarone: a common source of iodine-induced thyrotoxicosis. Horm Res 1987;26:158-71.
75. Martino E, Bartalena L, Bogazzi F and Braverman LE. The effects of amiodarone on the Thyroid. Endoc Rev 2001;22:240-54.
76. Daniels GH. Amiodarone-induced thyrotoxicosis. J Clin Endocrinol Metab 2001;86:3-8.
77. Harjai KJ and Licata AA. Effects of amiodarone on thyroid function. Ann Intern Med 1997;126:63-73.
78. Caron P. Effect of amiodarone on thyroid function. Press Med 1995;24:1747-51.
79. Bartalena L, Grasso L, Brogioni S, Aghini-Lombardi F, Braverman LE and Martino E. Serum interleukin-6 in amiodarone-induced thyrotoxicosis. J Clin Endocrinol Metab 1994;78:423-7.
80. Eaton SE, Euinton HA, Newman CM, Weetman AP and Bennet WM. Clinical experience of amiodarone-induced thyrotoxicosis over a 3-year period: role of colour-flow Doppler sonography. Clin Endocrinol 2002;56:33-8.
81. Lazarus JH. The effects of lithium therapy on thyroid and thyrotropin-releasing hormone. Thyroid 1998;8:909-13.
82. Kusalic M and Engelsmann F. Effect of lithium maintenance therapy on thyroid and parathyroid function. J Psych Neurosci 1999;24:227-33.
83. Oakley PW, Dawson AH and Whyte IM. Lithium: thyroid effects and altered renal handling. Clin Toxicol 2000;38:333-7.
84. Mendel CM, Frost PH, Kunitake ST and Cavalieri RR. Mechanism of the heparin-induced increase in the concentration of free thyroxine in plasma. J Clin Endocrinol Metab1987;65:1259-64.
85. Iitaka M, Kawasaki S, Sakurai S, Hara Y, Kuriyama R, Yamanaka K et al. Serum substances that interfere with thyroid hormone assays in patients with chronic renal failure. Clin Endocrinol 1998;48:739-46.
86. Bowie LJ, Kirkpatrick PB and Dohnal JC. Thyroid function testing with the TDx: Interference from endogenous fluorophore. Clin Chem 1987;33:1467.
87. DeGroot LJ and Mayor G. Admission screening by thyroid function tests in an acute general care teaching hospital. Amer J Med 1992;93:558-64.
88. Kaptein EM. Thyroid hormone metabolism and thyroid diseases in chronic renal failure. Endocr Rev 1996;17:45-63.
89. Van den Berghe G, De Zegher F and Bouillon R. Acute and prolonged critical illness as different neuroendocrine paradigms. J Clin Endocrinol Metab 1998;83:1827-34.
90. Van den Berhe G. Novel insights into the neuroendocrinology of critical illness. Eur J Endocrinol 2000;143:1-13.
91. Wartofsky L and Burman KD. Alterations in thyroid function in patients with systemic illness: the “euthyroid sick syndrome”. Endocrinol Rev 1982;3:164-217.- 114 –
92. Spencer CA, Eigen A, Duda M, Shen D, Qualls S, Weiss S et al. Sensitive TSH tests – specificity limitations for screening for thyroid disease in hospitalized patients. Clin Chem 1987;33:1391-1396.
93. Stockigt JR. Guidelines for diagnosis and monitoring of thyroid disease: nonthyroidal illness. Clin Chem 1996;42:188-92.
94. Nelson JC and Weiss RM. The effects of serum dilution on free thyroxine (T4) concentration in the low T4 syndrome of nonthyroidal illness. J Clin Endocrinol Metab 1985;61:239-46.
95. Chopra IJ, Huang TS, Beredo A, Solomon DH, Chua Teco GN. Serum thyroid hormone binding inhibitor in non thyroidal illnesses. Metabolism 1986;35:152-9.
96. Wang R, Nelson JC and Wilcox RB. Salsalate administration – a potential pharmacological model of the sick euthyroid syndrome. J Clin Endocrinol Metab 1998;83:3095-9.
97. Sapin R, Schliener JL, Kaltenbach G, Gasser F, Christofides N, Roul G et al. Determination of free triiodothyronine by six different methods in patients with non-thyroidal illness and in patients treated with amiodarone. Ann Clin Biochem 1995;32:314-24.
98. Docter R, van Toor H, Krenning EP, de Jong M and Hennemann G. Free thyroxine assessed with three assays in sera of patients with nonthyroidal illness and of subjects with abnormal concentrations of thyroxine-binding proteins. Clin Chem 1993;39:1668-74.
99. Wilcox RB, Nelson JC and Tomei RT. Heterogeneity in affinities of serum proteins for thyroxine among patients with non-thyroidal illness as indicated by the serum free thyroxine response to serum dilution. Eur J Endocrinol 1994;131:9-13.
100. Liewendahl K, Tikanoja S, Mahonen H, Helenius T, Valimaki M and Tallgren LG. Concentrations of iodothyronines in serum of patients with chronic renal failure and other nonthyroidal illnesses: role of free fatty acids. Clin Chem 1987;33:1382-6.
101. Sapin R, Schlienger JL,Gasser F, Noel E, Lioure B, Grunenberger F. Intermethod discordant free thyroxine measurements in bone marrow-transplanted patients. Clin Chem 2000;46:418-22.
102. Chopra IJ. Simultaneous measurement of free thyroxine and free 3,5,3′-triiodothyronine in undiluted serum by direct equilibrium dialysis/radioimmunoassay: evidence that free triiodothyronine and free thyroxine are normal in many patients with the low triiodothyronine syndrome. Thyroid 1998;8:249-57.
103. Hamblin PS, Dyer SA, Mohr VS, Le Grand BA, Lim C-F, Tuxen DB, Topliss DJ and Stockigt JR. Relationship between thyrotropin and thyroxine changes during recovery from severe hypothyroxinemia of critical illness. J Clin Endocrinol Metab 1986;62:717-22.
104. Brent GA and Hershman JM. Thyroxine therapy in patients with severe nonthyroidal illnesses and low serum thyroxine concentrations. J Clin Endocrinol Metab 1986;63:1-8.
105. De Groot LJ. Dangerous dogmas in medicine: the nonthyroidal illness syndrome. J Clin Endocrinol Metab 1999;84:151-64.
106. Burman KD and Wartofsky L. Thyroid function in the intensive care unit setting. Crit Care Clin 2001;17:43-57.
107. Behrend EN, Kemppainen RJ and Young DW. Effect of storage conditions on cortisol, total thyroxine and free thyroxine concentrations in serum and plasma of dogs. J Am Vet Med Assoc 1998;212:1564-8.
108. Oddie TH, Klein AH, Foley TP and Fisher DA. Variation in values for iodothyronine hormones, thyrotropin and thyroxine binding globulin in normal umbilical-cord serum with season and duration of storage. Clin Chem 1979;25:1251-3.
109. Koliakos G, Gaitatzi M and Grammaticos P. Stability of serum TSH concentration after non refrigerated storage. Minerva Endocrinol 1999;24:113-5.
110. Waite KV, Maberly GF and Eastman CJ. Storage conditions and stability of thyrotropin and thyroid hormones on filter paper. Clin Chem 1987;33:853-5.
111. Levinson SS. The nature of heterophilic antibodies and their role in immunoassay interference. J Clin Immunoassay 1992;15:108-15.
112. Norden AGM, Jackson RA, Norden LE, Griffin AJ, Barnes MA and Little JA. Misleading results for immunoassays of serum free thyroxine in the presence of rheumatoid factor. Clin Chem 1997;43:957-62.
113. Covinsky M, Laterza O, Pfeifer JD, Farkas-Szallasi T and Scott MG. Lambda antibody to Esherichia coli produces false-positive results in multiple immunometric assays. Clin Chem 2000;46:1157-61.
114. Martel J, Despres N, Ahnadi CE, Lachance JF, Monticello JE, Fink G, Ardemagni A, Banfi G, Tovey J, Dykes P, John R, Jeffery J and Grant AM. Comparative multicentre study ofa panel of thyroid tests using different automated immunoassay platforms and specimens at high risk of antibody interference. Clin Chem Lab Med 2000;38:785-93.
115. Howanitz PJ, Howanitz JH, Lamberson HV and Ennis KM. Incidence and mechanism of spurious increases in serum Thyrotropin. Clin Chem 1982;28:427-31.
116. Boscato, L. M. and M. C. Stuart. Heterophilic antibodies: a problem for all immunoassays. Clin Chem 1988;34:27-33.
117. Kricka LJ. Human anti-animal antibody interference in immunological assays. Clin Chem 1999;45:942-56.
118. Sapin R and Simon C. False hyperprolactinemia corrected by the use of heterophilic antibody-blocking agent. Clin Chem 2001;47:2184-5.
119. Feldt-Rasmussen U, Petersen PH, Blaabjerg O and Horder M. Long-term variability in serum thyroglobulin and thyroid related hormones in healthy subjects. Acta Endocrinol (Copenh) 1980;95:328-34.
120. Browning MCK, Ford RP, Callaghan SJ and Fraser CG. Intra-and interindividual biological variation of five analytes used in assessing thyroid function: implications for necessary standards of performance and the interpretation of results. Clin Chem 1986;32:962-6.
121. Lum SM and Nicoloff JT. Peripheral tissue mechanism for maintenance of serum triiodothyronine values in a thyroxine-deficient state in man. J Clin Invest 1984;73:570-5.
122. Spencer CA and Wang CC. Thyroglobulin measurement:- Techniques, clinical benefits and pitfalls. Endocrinol Metab Clin N Amer 1995;24:841-63.
123. Weeke J and Gundersen HJ. Circadian and 30 minute variations in serum TSH and thyroid hormones in normal subjects. Acta Endocrinol 1978;89:659-72.
124. Brabant G, Prank K, Hoang-Vu C and von zur Muhlen A. Hypothalamic regulation of pulsatile thyrotropin secretion. J Clin Endocrinol Metab 1991;72:145-50.
125. Fraser CG, Petersen PH, Ricos C and Haeckel R. Proposed quality specifications for the imprecision and inaccuracy of analytical systems for clinical chemistry. Eur J Clin Chem Biochem 1992;30:311-7.
126. Rodbard, D. Statistical estimation of the minimal detectable concentration (“sensitivity”) for radioligand assays. Anal Biochem 1978;90:1-12.
127. Ekins R and Edwards P. On the meaning of “sensitivity”. Clin Chem 1997;43:1824-31.
128. Fuentes-Arderiu X and Fraser CG. Analytical goals for interference. Ann Clin Biochem 1991;28:393-5.
129. Petersen PH, Fraser CG, Westgard JO and Larsen ML. Analytical goal-setting for monitoring patients when two analytical methods are used. Clin Chem 1992;38:2256-60.
130. Fraser CG and Petersen PH. Desirable standards for laboratory tests if they are to fulfill medical needs. Clin Chem 1993;39:1453-5.
131. Stockl D, Baadenhuijsen H, Fraser CG, Libeer JC, Petersen PH and Ricos C. Desirable routine analytical goals for quantities assayed in serum. Discussion paper from the members of the external quality assessment (EQA) Working Group A on analytical goals in laboratory medicine. Eur J Clin Chem Clin Biochem 1995;33:157-69.
132. Plebani M, Giacomini A, Beghi L, de Paoli M, Roveroni G, Galeotti F, Corsini A and Fraser CG. Serum tumor markers in monitoring patients: interpretation of results using analytical and biological variation. Anticancer Res 1996;16:2249-52.
133. Browning MC, Bennet WM, Kirkaldy AJ and Jung RT. Intra-individual variation of thyroxin, triiodothyronine and thyrotropin in treated hypothyroid patients: implications for monitoring replacement therapy. Clin Chem 1988;34:696-9.
134. Harris EK. Statistical principles underlying analytic goal-setting in clinical chemistry. Am J Clin Pathol 1979;72:374-82.
135. Nelson JC and Wilcox RB. Analytical performance of free and total thyroxine assays. Clin Chem 1996;42:146-54.
136. Evans SE, Burr WA and Hogan TC. A reassessment of 8-anilino-1-napthalene sulphonic acid as a thyroxine binding inhibitor in the radioimmunoassay of thyroxine. Ann Clin Biochem 1977;14:330-4.
137. Karapitta CD, Sotiroudis TG, Papadimitriou A and Xenakis A. Homogeneous enzyme immunoassay for triiodothyronine in serum. Clin Chem 2001;47:569-74.
138. De Brabandere VI, Hou P, Stockl D, Theinpont LM and De Leenheer AP. Isotope dilution-liquid chromatography/electrospray ionization-tandem mass spectrometry for the determination of serum thyroxine as a potential reference method. Rapid Commun Mass Spectrom 1998;12:1099-103.
139. Tai SSC, Sniegoski LT and Welch MJ. Candidate reference method for total thyroxine in human serum: Use of isotope-dilution liquid chromatography-mass spectrometry with electrospray ionization. Clin Chem 2002;48:637-42.
140. Thienpont LM, Fierens C, De Leenheer AP and Przywara L. Isotope dilution-gas chromatography/mass spectrometry and liquid chromatography/electro-spray ionization-tandem mass spectrometry for the determination of triiodo-L-thyronine in serum. Rapid Commun Mass Spectrom 1999;13:1924-31.
141. Sarne DH, Refetoff S, Nelson JC and Linarelli LG. A new inherited abnormality of thyroxine-binding globulin (TBG-San Diego) with decreased affinity for thyroxine and triiodothyronine. J Clin Endocrinol Metab 1989;68:114-9.
142. Schussler GC. The thyroxine-binding proteins. Thyroid 2000;10:141-9.
143. Beck-Peccoz P, Romelli PB, Cattaneo MG, Faglia G, White EL, Barlow JW et al. Evaluation of free T4 methods in the presence of iodothyronine autoantibodies. J Clin Endocrinol Metab 1984;58:736-9.
144. Sakata S, Nakamura S and Miura K. Autoantibodies against thyroid hormones or iodothyronine. Ann Intern Med 1985;103:579-89.
145. Despres N and Grant AM. Antibody interference in thyroid assays: a potential for clinical misinformation. Clin Chem 1998;44:440-54.
146. Hay ID, Bayer MF, Kaplan MM, Klee GG, Larsen PR and Spencer CA. American Thyroid Association Assessment of Current Free Thyroid Hormone and Thyrotropin Measurements and Guidelines for Future Clinical Assays. Clin Chem 1991;37:2002 – 2008.
147. Ekins R. The science of free hormone measurement. Proc UK NEQAS Meeting 1998;3:35-59.
148. Wang R, Nelson JC, Weiss RM and Wilcox RB. Accuracy of free thyroxine measurements across natural ranges of thyroxine binding to serum proteins. Thyroid 2000;10:31-9.
149. Nelson JC, Wilcox BR and Pandian MR. Dependence of free thyroxine estimates obtained with equilibrium tracer dialysis on the concentration of thyroxine-binding globulin. Clin Chem 1992;38:1294-1300.
150. Ekins R. The free hormone hypothesis and measurement of free hormones. Clin Chem 1992;38:1289-93.
151. Ekins RP. Ligand assays: from electrophoresis to miniaturized microarrays. Clin Chem 1998;44:2015-30.
152. Ekins R. Analytic measurements of free thyroxine. Clin Lab Med 1993;13:599-630.
153. Nusynowitz, M. L. Free-thyroxine index. JAMA 1975;232:1050.
154. Larsen PR, Alexander NM, Chopra IJ, Hay ID, Hershman JM, Kaplan MM et al. Revised nomenclature for tests of thyroid hormones and thyroid-related proteins in serum. J Clin Endocrinol Metab 1987;64:1089-94.
155. Burr WA, Evans SE, Lee J, Prince HP, Ramsden DB. The ratio of thyroxine to thyroxine-binding globulin measurement in the evaluation of thyroid function. Clin Endocrinol 1979;11:333-42.
156. Attwood EC and Atkin GE. The T4: TBG ratio: a re-evaluation with particular reference to low and high serum TBG levels. Ann Clin Biochem 1982;19:101-3.
157. Szpunar WE, Stoffer SS and DiGiulio W. Clinical evaluation of a thyroxine binding globulin assay in calculation a free thyroxine index in normal, thyroid disease and sick euthyroid patients. J Nucl Med 1987;28:1341-3.
158. Nelson JC and Tomei RT. Dependence of the thyroxin/thyroxin-binding globulin (TBG) ratio and the free thyroxin index on TBG concentrations. Clin Chem 1989;35:541-4.
159. Sterling K and Brenner MA. Free thyroxine in human serum: Simplified measurement with the aid of magnesium precipitation. J Clin Invest 1966;45:153-60.
160. Schulssler GC and Plager JE. Effect of preliminary purification of 131-Thyroxine on the determination of free thyroxine in serum. J Clin Endocrinol 1967;27:242-50.
161. Nelson JC and Tomei RT. A direct equilibrium dialysis/radioimmunoassay method for the measurement of free thyroxin in undiluted serum. Clin Chem 1988;34:1737-44.
162. Tikanoja SH. Ultrafiltration devices tested for use in a free thyroxine assay validated by comparison with equilibrium dialysis. Scand J Clin Lab Invest 1990;50:663-9.
163. Ellis SM and Ekins R. Direct measurement by radioimmunoassay of the free thyroid hormone concentrations in serum. Acta Endocrinol (Suppl) 1973;177:106-110.
164. Weeke J and Orskov H. Ultrasensitive radioimmunoassay for direct determination of free triiodothyronine concentration in serum. Scand J Clin Lab Invest 1975;35:237-44.
165. Surks MI, Hupart KH, Chao P and Shapiro LE. Normal free thyroxine in critical nonthyroidal illnesses measured by ultrafiltration of undiluted serum and equilibrium dialysis. J Clin Endocrinol Metab 1988;67:1031-9.
166. Holm SS andreasen L, Hansen SH, Faber J and Staun-Olsen P. Influence of adsorption and deproteination on potential free thyroxine reference methods. Clin Chem 2002;48:108-114.
167. Jaume JC, Mendel CM, Frost PH,Greenspan FS, Laughton CW. Extremely low doses of heparin release lipase activity into the plasma and can thereby cause artifactual elevations in the serum-free thyroxine concentrations as measured by equilibrium dialysis. Thyroid 1996;6:79-83.
168. Stevenson HP, Archbold GP, Johnston P, Young IS, Sheridan B. Misleading serum free thyroxine results during low molecular weight heparin treatment. Clin Chem 1998;44:1002-7.
169. Laji K, Rhidha B, John R, Lazarus J and Davies JS. Artifactual elevations in serum free thyroxine and triiodothyronine concentrations during heparin therapy. QJM 2001;94:471-3.
170. Lim CF, Bai Y, Topliss DJ, Barlow JW and Stockigt JR. Drug and fatty acid effects on serum thyroid hormone binding. J Clin Endocrinol Metab 1988;67:682-8.
171. Czako, G., M. H. Zweig, C. Benson and M. Ruddel. On the albumin-dependence of measurements of free thyroxin. II Patients with non-thyroidal illness. Clin Chem 1987;33:87-92.
172. Csako G, Zwieg MH, Glickman J, Ruddel M and K. J. Direct and indirect techniques for free thyroxin compared in patients with nonthyroidal illness. II. Effect of prealbumin, albumin and thyroxin-binding globulin. Clin Chem 1989;35:1655-62.
173. Csako G, Zweig MH, Glickman J, Kestner J and Ruddel M. Direct and indirect techniques for free thyroxin compared in patients with nonthyroidal illness. I. Effect of free fatty acids. Clin Chem 1989;35:102-9.
174. Ross HA and Benraad TJ. Is free thyroxine accurately measurable at room temperature? Clin Chem 1992;38:880-6.
175. Van der Sluijs Veer G, Vermes I, Bonte HA and Hoorn RKJ. Temperature effects on Free Thyroxine Measurement: Analytical and Clinical Consequences. Clin Chem 1992;38:1327-31.
176. Fisher DA. The hypothyroxinemia of prematurity. J Clin Endocrinol Metab 1997;82:1701-3.
177. Stockigt JR, Stevens V, White EL and Barlow JW. Unbound analog radioimmunoassays for free thyroxin measure the albumin-bound hormone fraction. Clin Chem 1983;29:1408-10.
178. Aravelo G. Prevalence of familial dysalbuminemic hyperthyroxinemia in serum samples received for thyroid testing. Clin Chem 1991;37:1430-1.
179. Sapin R and Gasser F. Anti-solid phase antibodies interfering in labeled-antibody assays for free thyroid hormones. Clin Chem 1995;45:1790-1.
180. Inada M and Sterling K. Thyroxine transport in thyrotoxicosis and hypothyroidism. J Clin Invest 1967;46:1442-50.
181. Lueprasitsakul W, Alex S, Fang SL, Pino S, Irmscher K, Kohrle J et al. Flavonoid administration immediately displaces thyroxine (T4) from serum transthyretin, increases serum free T4 and decreases serum thyrotropin in the rat. Endocrinol 1990;126:2890-5.
182. Stockigt JR, Lim CF, Barlow J, Stevens V, Topliss DJ, Wynne KN. High concentrations of furosemide inhibit plasma binding of thyroxine. J Clin Endocrinol Metab 1984;59:62-6.
183. Hawkins RC. Furosemide interference in newer free thyroxine assays. Clin Chem 1998;44:2550-1.
184. Wang R, Nelson JC and Wilcox RB. Salsalate and salicylate binding to and their displacement of thyroxine from thyroxine-binding globulin, transthyrin and albumin. Thyroid 1999;9:359-64.
185. Munro SL, Lim C-F, Hall JG, Barlow JW, Craik DJ, Topliss DJ and Stockigt JR. Drug competition for thyroxine binding to transthyretin (prealbumin): comparison with effects on thyroxine-binding globulin. J Clin Endocrinol Metab 1989;68:1141-7.
186. Stockigt JR, Lim C-F, Barlow JW and Topliss DJ. 1997. Thyroid hormone transport. Springer Verlag, Heidelberg. 119 pp.
187. Surks MI and Defesi CR. Normal free thyroxine concentrations in patients treated with phenytoin or carbamazepine: a paradox resolved. JAMA 1996;275:1495-8.
188. Ross HA. A dialysis method for the measurement of free iodothyronine and steroid hormones in blood. Experientia 1978;34:538-9.
189. Sapin R. Serum thyroxine binding capacity-dependent bias in five free thyroxine immunoassays: assessment with serum dilution experiments and impact on diagnostic performance. Clin Biochem 2001;34:367-71.
190. Law LK, Cheung CK and Swaminathan R. Falsely high thyroxine results by fluorescence polarization in sera with high background fluorescence. Clin Chem 1988;34:1918.
191. Kricka LJ. Interferences in Immunoassay – still a threat. Clin Chem 2000;46:1037-8.
192. McBride JH, Rodgerson DO and Allin RE. Choriogonadotrophin interference in a sensitive assay for Thyrotropin. Clin Chem 1987;33:1303-4.
193. Ritter D, Stott R, Grant N and Nahm MH. Endogenous antibodies that interfere with Thyroxine fluorescence polarization assay but not with radioimmunoassay or EMIT. Clin Chem 1993;39:508-11.
194. DeGroot LJ, Larsen PR, Refetoff S and Stanbury JB. The Thyroid and its Diseases. Fifth Edition, 1984;John Wiley & Sons, Inc., New York:266-7.
195. Beck-Peccoz P, Amr S, Menezes-Ferreira NM, Faglia G and Weintraub BD. Decreased receptor binding of biologically inactive thyrotropin in central hypothyroidism: effect of treatment with thyrotropin-releasing hormone. N Engl J Med 1985;312:1085-90.
196. Beck-Peccoz P and Persani L. Variable biological activity of thyroid-stimulating hormone. Eur J Endocrinol 1994;131:331-40.
197. Persani L, Ferretti E, Borgato S, Faglia G and Beck-Peccoz P. Circulating thyrotropin bioactivity in sporadic central hypothyroidism. J Clin Endocrinol Metab 2000;85:3631-5.
198. Rafferty B and Gaines Das R. Comparison of pituitary and recombinant human thyroid-stimulating hormone (rhTSH) in a multicenter collaborative study: establishment of the first World Health Organization reference reagent for rhTSH. Clin Chem 1999;45:2207-15.
199. Persani L, Borgato S, Romoli R, Asteria C, Pizzocaro A and Beck-Peccoz P. Changes in the degree of sialylation of carbohydrate chains modify the biological properties of circulating thyrotropin isoforms in various physiological and pathological states. J Clin Endocrinol Metab 1998;83:2486-92.
200. Gershengorn MC and Weintraub BD. Thyrotropin-induced hyperthyroidism caused by selective pituitary resistance to thyroid hormone. A new syndrome of “inappropriate secretion of TSH”. J Clin Invest 1975;56:633-42.
201. Faglia G, Beck-Peccoz P, Piscitelli G and Medri G. Inappropriate secretion of thyrotropin by the pituitary. Horm Res 1987;26:79-99.
202. Spencer CA, Takeuchi M and Kazarosyan M. Current status and performance goals for serum thyrotropin (TSH) assays. Clinical Chemistry 1996;42:141-145.
203. Laurberg P. Persistent problems with the specificity of immunometric TSH assays. Thyroid 1993;3:279-83.
204. Spencer CA, Schwarzbein D, Guttler RB, LoPresti JS and Nicoloff JT. TRH stimulation test responses employing third and fourth generation TSH assays. J Clin Endocrinol Metab 1993;76:494-498.
205. Vogeser M, Weigand M, Fraunberger P, Fischer H and Cremer P. Evaluation of the ADVIA Centaur TSH-3 assay. Clin Chem Lab Med 2000;38:331-4.
206. Spencer CA, Takeuchi M, Kazarosyn M, MacKenzie F, Beckett GJ and Wilkinson E. Interlaboratory/intermethod differences in functional sensitivity of immunometric assays for thyrotropin (TSH): impact on reliability of measurement of subnormal concentration. Clin Chem 1995;41:367-74.
207. Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, Evans JG, Young E, Bird T and Smith PA. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol 1977;7:481-93.
208. Rago T, Chiovato L, Grasso L, Pinchera A and Vitti P. Thyroid ultrasonography as a tool for detecting thyroid autoimmune diseases and predicting thyroid dysfunction in apparently healthy subjects. J Endocrinol Invest 2001;24:763-9.
209. Hershman JM and Pittman JA. Utility of the radioimmunoassay of serum thyrotropin in man. Ann Intern Med 1971;74:481-90.
210. Becker DV, Bigos ST, Gaitan E, Morris JC, Rallison ML, Spencer CA, Sugawara M, Middlesworth LV and Wartofsky L. Optimal use of blood tests for assessment of thyroid function. JAMA 1993;269:2736.
211. Canaris GJ, Manowitz NR, Mayor G and Ridgway EC. The Colorado Thyroid Disease Prevalence Study. Arch Intern Med 2000;160:19-27.
212. Skamene A and Patel YC. Infusion of graded concentrations of somatostatin in man: pharmacokinetic and differential inhibitory effects on pituitary and islet hormones. Clin Endocrinol 1984;20:555-64.
213. Berghout A, Wiersinga WM, Smits NJ and Touber JL. Interrelationships between age, thyroid volume, thyroid nodularity and thyroid function in patients with sporadic nontoxic goiter. Am J Med 1990;89:602-8.
214. Parle JV, Franklyn JA, Cross KW, Jones SC and Sheppard MC. Prevalence and follow-up of abnormal thyrotropin (TSH) concentrations in the elderly in the United Kingdom. Clin Endocrinol 1991;34:77-83.
215. Danese D, Sciacchitano S, Farsetti A Andreoli M and Pontecorvi A. Diagnostic accuracy of conventional versus sonography-guided fine-needle aspiration biopsy of thyroid nodules. Thyroid 1998;8:15-21.
216. McDermott MT and Ridgway EC. Subclinical hypothyroidism is mild thyroid failure and should be treated. J Clin Endocrinol Metab 2001;86:4585-90.
217. Chu JW and Crapo LM. The treatment of subclinical hypothyroidism is seldom necessary. J Clin Endocrinol Metab 2001;86:4591-9.
218. Lewis GF, Alessi CA, Imperial JG and Refetoff S. Low serum free thyroxine index in ambulating elderly is due to a resetting of the threshold of thyrotropin feedback suppression. JCEM 1991;73:843-9.
219. Pearce CJ and Himsworth RL. Total and free thyroid hormone concentrations in patients receiving maintenance replacement treatment with thyroxine. Br Med J 1984;288:693-5.
220. Fish LH, Schwarz HL, Cavanaugh MD, Steffes MW, Bantle JP, Oppenheimer JH. Replacement dose, metabolism and bioavailability of levothyroxine in the treatment of hypothyroidism. N Engl J Med 1987;316:764-70.
221. Sawin CT, Herman T, Molitch ME, London MH and Kramer SM. Aging and the thyroid. Decreased requirement for thyroid hormone in older hypothyroid patients. Amer J Med 1983;75:206-9.
222. Davis FB, LaMantia RS, Spaulding SW, Wemann RE and Davis PJ. Estimation of a physiologic replacement dose of levothyroxine in elderly patients with hypothyroidism. Arch Intern Med 1984;144.
223. Arafah BM. Estrogen therapy may necessitate an increase in thyroxine dose for hypothyroidism. NEJM 2001;344:1743-9.
224. Scheithauer BW, Kovacs K, Randall RV and Ryan N. Pituitary gland in hypothyroidism. Histologic and immunocytologic study. Arch Pathol Lab Med 1985;109:499-504.
225. Ain KB, Pucino F, Shiver T and Banks SM. Thyroid hormone levels affected by time of blood sampling in thyroxine-treated patients. Thyroid 1993;3:81-5.
226. Chorazy PA, Himelhoch S, Hopwood NJ, Greger NG and Postellon DC. Persistent hypothyroidism in an infant receiving a soy formula: case report and review of the literature. Pediatrics 1995;96:148-50.
227. Dulgeroff AJ and Hershman JM. Medical therapy for differentiated thyroid carcinoma. Endocrinol Rev 1994;15:500-15.
228. Pujol P, Daures JP, Nsakala N, Baldet L, Bringer J and Jaffiol C. Degree of thyrotropin suppression as a prognostic determinant in differentiated thyroid cancer. J Clin Endocrinol Metab 1996;81:4318-23.
229. Cooper DS, Specker B, Ho M, Sperling M, Ladenson PW, Ross DS, Ain KB, Bigos ST, Brierley JD, Haugen BR, Klein I, Robbins J, Sherman SI, Taylor T and Maxon HR 3rd. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National thyroid Cancer Treatment Cooperative Registry. Thyroid
1999;8:737-44.
230. Hurley DL and Gharib H. Evaluation and management of multinodular goiter. Otolaryngol Clin North Am 1996;29:527-40.
231. Bayer MF, Macoviak JA and McDougall IR. Diagnostic performance of sensitive measurements of serum thyrotropin during severe nonthyroidal illness: Their role in the diagnosis of hyperthyroidism. Clin Chem 1987;33:2178-84.
232. Lum SM, Kaptein EM and Nicoloff JT. Influence of nonthyroidal illnesses on serum thyroid hormone indices in hyperthyroidism. West J Med 1983;138:670-5.
233. Faglia G, Bitensky L, Pinchera A, Ferrari C, Paracchi A, Beck-Peccoz P, Ambrosi B and Spada A. Thyrotropin secretion in patient with central hypothyroidism: Evidence for reduced biological activity of immunoreactive thyrotropin. J Clin Endocrinol Metab 1979;48:989-98.
234. Faglia G, Beck-Peccoz P, Ballabio M and Nava C. Excess of beta-subunit of thyrotropin (TSH) in patients with idiopathic central hypothyroidism due to the secretion of TSH with
reduced biological activity. J Clin Endocrinol Metab 1983;56:908-14.
235. Faglia G. The clinical impact of the thyrotropin-releasing hormone test. Thyroid 1998;8:903-8.
236. Trejbal D, Sulla I, Trejbalova L, Lazurova I, Schwartz P and Machanova Y. Central hypothyroidism – various types of TSH responses to TRH stimulation. Endocr Regul 1994;28:35-40.
237. Faglia G, Ferrari C, Paracchi A, Spada A and Beck-Peccoz P. Triiodothyronine response to thyrotropin releasing hormone in patients with hypothalamic-pituitary disorders. Clin Endocrinol 1975;4:585-90.
238. Horimoto M, Nishikawa M, Ishihara T, Yoshikawa N, Yoshimura M and Inada M. Bioactivity of thyrotropin (TSH) in patients with central hypothyroidism: comparison between in vivo 3,5,3′-triiodothyronine response to TSH and in vitro bioactivity of TSH. J Clin Endocrinol Metab 1995;80:1124-8.
239. Refetoff S, Weiss RE and Usala SJ. The syndromes of resistance to thyroid hormone. Endocr Rev 1993;14:348-99.
240. Weiss RE, Hayashi Y, Nagaya T, Petty KJ, Murata Y, Tunca H, Seo H and Refetoff S. Dominant inheritance of resistance to thyroid hormone not linked to defects in the thyroid hormone receptors alpha or beta genes may be due to a defective co-factor. J Clin Endocrinol Metab 1996;81:4196-203.
241. Snyder D, Sesser D, Skeels M et al. Thyroid disorders in newborn infants with elevated screening T4. Thyroid 1997;7 (Suppl 1):S1-29 (abst).
242. Refetoff S. 2000. Resistance to Thyroid Hormone. In The Thyroid. Braverman LE and Utiger RD, editor. Lippincott Williams & Wilkins, Philadelphia. 1028-43.
243. Beck-Peccoz P and Chatterjee VKK. The variable clinical phenotype in thyroid hormone resistance syndrome. Thyroid 1994;4:225-32.
244. Persani L, Asteria C, Tonacchera M, Vitti P, Krishna V, Chatterjee K and Beck-Peccoz P. Evidence for the secretion of thyrotropin with enhanced bioactivity in syndromes of thyroid hormone resistance. J Clin Endocrinol Metab 1994;78:1034-9.
245. Sarne DH, Sobieszczyk S, Ain KB and Refetoff S. Serum thyrotropin and prolactin in the syndrome of generalized resistance to thyroid hormone: responses to thyrotrophin releasing hormone stimulation and triiodothyronine suppression. J Clin Endocrinol Metab 1990;70:1305-11.
246. Ercan-Fang S, Schwartz HL, Mariash CN and Oppenheimer JH. Quantitative assessment of pituitary resistance to thyroid hormone from plots of the logarithm of thyrotropin versus serum free thyroxine index. J Clin Endocrinol Metab 2000;85:2299-303.
247. Safer JD, Colan SD, Fraser LM and Wondisford FE. A pituitary tumor in a patient with thyroid hormone resistance: a diagnostic dilemma. Thyroid 2001;11:281-91.
248. Marcocci C and Chiovato L. 2000. Thyroid -directed antibodies. In Thyroid. B. L. a. U. RD, editor. Lippincott Williams and Wilkins, Philadelphia. 414-31.
249. Chiovato L, Bassi P, Santini F, Mammoli C, Lapi P, Carayon P and Pinchera A. Antibodies producing complement-mediated thyroid cytotoxicity in patients with atrophic or goitrous autoimmune thyroiditis. J Clin Endocrinol Metab 1993;77:1700-5.
250. Guo J, Jaume JC, Rapoport B and McLachlan SM. Recombinant thyroid peroxidase-specific Fab converted to immunoglobulin G (IgG)molecules: evidence for thyroid cell damage by IgG1, but not IgG4, autoantibodies. J Clin Endocrinol Metab 1997;82:925-31.
251. Doullay F, Ruf J, Codaccioni JL and Carayon P. Prevalence of autoantibodies to thyroperoxidase in patients with various thyroid and autoimmune diseases. Autoimmunity 1991;9:237-44.
252. Radetti G, Persani L, Moroder W, Cortelazzi D, Gentili L, Beck-Peccoz P. Transplacental passage of anti-thyroid autoantibodies in a pregnant woman with auto-immune thyroid disease. Prenatal Diagnosis 1999;19:468-71.
253. Heithorn R, Hauffa BP and Reinwein D. Thyroid antibodies in children of mothers with autoimmune thyroid disorders. Eur J Pediatr 1999;158:24-8.
254. Feldt-Rasmussen. Anti-thyroid peroxidase antibodies in thyroid disorders and non thyroid autoimmune diseases. Autoimmunity 1991;9:245-51.
255. Mariotti S, Chiovato L, Franceschi C and Pinchera A. Thyroid autoimmunity and aging. Exp Gerontol 1999;33:535-41.
256. Ericsson UB, Christensen SB and Thorell JI. A high prevalence of thyroglobulin autoantibodies in adults with and without thyroid disease as measured with a sensitive solid-phase immunosorbent radioassay. Clin Immunol Immunopathol 1985;37:154-62.
257. Feldt-Rasmussen U, Hoier-Madsen M, Rasmussen NG, Hegedus L and Hornnes P. Anti-thyroid peroxidase antibodies during pregnancy and postpartum. Relation to postpartum thyroiditis. Autoimmunity 1990;6:211-4.
258. Premawardhana LD, Parkes AB, AMMARI F, John R, Darke C, Adams H and Lazarus JH. Postpartum thyroiditis and long-term thyroid status: prognostic influence of Thyroid Peroxidase Antibodies and ultrasound echogenicity. J Clin Endocrinol Metab 2000;85:71-5.
259. Johnston AM and Eagles JM. Lithium-associated clinical hypothyroidism. Prevalence and risk factors. Br. J Psychiatry 1999;175:336-9.
260. Bell TM, Bansal AS, Shorthouse C, Sandford N and Powell EE. Low titre autoantibodies predict autoimmune disease during interferon alpha treatment of chronic hepatitis C. J Gastroenterol Hepatol 1999;14:419-22.
261. Ward DL and Bing-You RG. Autoimmune thyroid dysfunction induced by interfereon-alfa treatment for chronic hepatitis C: screening and monitoring recommendations. Endoc Pract 2001;7:52-8.
262. Carella C, Mazziotti G, Morisco F, Manganella G, Rotondi M, Tuccillo C, Sorvillo F, Caporaso N and Amato G. Long-term outcome of interferon-alpha-induced thyroid autoimmunity and prognostic influence of thyroid autoantibody pattern at the end of treatment. J Clin Endocrinol Metab 2001;86:1925-9.
263. Feldt-Rasmussen U, Schleusener H and Carayon P. Meta-analysis evaluation of the impact of thyrotropin receptor antibodies on long term remission after medical therapy of Graves’ disease. J Clin Endocrinol Metab 1994;78:98-103.
264. Estienne V, Duthoit C, Di Costanzo, Lejeune PJ, Rotondi M, Kornfeld S et al. Multicenter study on TGPO autoantibodies prevalence in various thyroid and non-thyroid diseases: relationships with thyroglobulin and thyroperoxidase autoantibody parameters. Eur J Endocrinol 1999;141:563-9.
265. Czarnocka B, Ruf J, Ferrand M et al. Purification of the human thyroid peroxidase and its identification as the microsomal antigen involved in autoimmune thyroid diseases. FEBS Lett 1985;190:147-52.
266. Mariotti S, Caturegli P, Piccolo P, Barbesino G and Pinchera A. Antithyroid peroxidase autoantibodies in thyroid diseases. J Clin Endocrinol Metab 1990;71:661-9.
267. Rubello D, Pozzan GB, Casara D, Girelli ME, Boccato s, Rigon F, Baccichetti C, Piccolo M, Betterle C and Busnardo B. Natural course of subclinical hypothyroidism in Down’s syndrome: prospective study results and therapeutic considerations. J Endocrinol Invest 1995;18:35-40.
268. Karlsson B, Gustafsson J, Hedov G, Ivarsson SA and Anneren G. Thyroid dysfunction in Down’s syndrome: relation to age and thyroid autoimmunity. Arch Dis Child 1998;79:242-5.
269. Bussen S, Steck T and Dietl J. Increased prevalence of thyroid antibodies in euthyroid women with a history of recurrent in-vitro fertilization failure. Hum Reprod 2000;15:545-8.
270. Phan GQ, Attia P, Steinberg SM, White DE and Rosenberg SA. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol 2001;19:3477-82.
271. Durelli L, Ferrero B, Oggero A, Verdun E, Ghezzi A, Montanari E and Zaffaroni M. Thyroid function and autoimmunity during interferon-Beta-1b Treatment: a Multicenter Prospective Study. J Clin Endocrinol Metab 2001;86:3525-32.
272. Roti E, Minelli R, Giuberti T, Marchelli C, Schianchi C, Gardini E, Salvi M, Fiaccadori F, Ugolotti G, Neri TM and Braverman LE. Multiple changes in thyroid function in patients with chronic active HCV hepatitis treated with recombinant interferon-alpha. Am J Med 1996;101:482-7.
273. Ruf J, Carayon P and Lissitzky S. Various expression of a unique anti-human thyroglobulin antibody repertoire in normal state and autoimmune disease. Eur J Immunol 1985;15:268-72.
274. Ruf J, Toubert ME, Czarnocka B, Durand-Gorde JM,Ferrand M, Carayon P. Relationship between immunological structure and biochemical properties of human thyroid peroxidase. Endocrinol 1989;125:1211-8.
275. Feldt-Rasmussen U and Rasmussen A K. Serum thyroglobulin (Tg)in presence of thyroglobulin autoantibodies (TgAb). Clinical and methodological relevance of the interaction between Tg and TgAb in vivo and in vitro. J Endocrinol Invest 1985;8:571-6.
276. Spencer CA, Wang C, Fatemi S, Guttler RB, Takeuchi M and Kazarosyan M. Serum Thyroglobulin Autoantibodies: Prevalence, influence on serum thyroglobulin measurement and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab 1998;83:1121-7.
277. Pacini F, Mariotti S, Formica N and Elisei R. Thyroid autoantibodies in thyroid cancer: Incidence and relationship with tumor outcome. Acta Endocrinol 1988;119:373-80.
278. Rubello D, Casara D, Girelli ME, Piccolo M and Busnardo B. Clinical meaning of circulating antithyroglobulin antibodies in differentiated thyroid cancer: a prospective study. J Nucl Med 1992;33:1478-80.
279. Nordyke RA, Gilbert FI, Miyamoto LA and Fleury KA. The superiority of antimicrosomal over antithyroglobulin antibodies for detecting Hashimoto’s thyroiditis. Arch Intern Med 1993;153:862-5.
280. Di Cerbo A, Di Paoloa R, Menzaghi C, De Filippis V, Tahara K, Corda D et al. Graves’ immunoglobulins activate phospholipase A2 by recognizing specific epitopes on the thyrotropin receptor. J Clin Endocrinol Metab 1999;84:3283-92.
281. Kung AWC, Lau KS and Kohn LD. Epitope mapping of TSH Receptor-blocking antibodies in Graves’ disease that appear during pregnancy. J Clin Endocrinol Metab 2001;86:3647-53.
282. Ueta Y, Fukui H, Murakami M, Yamanouchi Y, Yamamoto R, Murao A et al. Development of primary hypothyroidism with the appearance of blocking-type antibody to thyrotropin receptor in Graves’ disease in late pregnancy. Thyroid 1999;9:179-82.
283. Gupta MK. Thyrotropin-receptor antibodies in thyroid diseases: advances in detection techniques and clinical application. Clin Chem Acta 2000;293:1-29.
284. Kung AW, Lau KS and Kohn LD. Characterization of thyroid-stimulating blocking antibodies that appeared during transient hypothyroidism after radioactive iodine therapy. Thyroid 2000;10:909-17.
285. Filetti S, Foti D, Costante G and Rapoport B. Recombinant human thyrotropin (TSH) receptor in a radioreceptor assay for the measurement of TSH receptor antibodies. J Clin Endocrinol Metab 1991;72:1096-101.
286. Adams DD and Purves HD. Abnormal responses in the assay of thyrotropin. Proc Univ Otago Med Sch 1956;34:11-12.
287. Morgenthaler NG. New assay systems for thyrotropin receptor antibodies. Current Opinion Endocrinol Diabetes 1998;6:251-60.
288. Kamijo K, Nagata A and Sato Y. Clinical significance of a sensitive assay for thyroid-stimulating antibodies in Graves’ disease using polyethylene glycol at high concentration and porcine thyroid cells. Endocrinol J 1999;46:397-403.
289. Takasu N, Yamashiro K, Ochi Y, Sato Y, Nagata A, Komiya I et al. TSBAb (TSH-Stimulation Blocking Antibody) and TSAb (Thyroid Stimulating Antibody) in TSBAb-positive patients with hypothyroidism and Graves’ patients with hyperthyroidism. Horm Metab Res 2001;33:232-7.
290. Costagliola S, Swillens S, Niccoli P, Dumont JE, Vassart G and Ludgate M. Binding assay for thyrotropin receptor autoantibodies using the recombinant receptor protein. J Clin Endocrinol Metab 1992;75:1540-44.
291. Morgenthaler NG, Hodak K, Seissler J, Steinbrenner H, Pampel I, Gupta M et al. Direct binding of thyrotropin receptor autoantibody to in vitro translated thyrotropin receptor: a comparison to radioreceptor assay and thyroid stimulating bioassay. Thyroid 1999;9:466-75.
292. Akamizu T, Inoue D, Kosugi S, Kohn LD and Mori T. Further studies of amino acids (268-304) in thyrotropin (TSH)-lutropin/chorionic gonadotropin (LH/CG) receptor chimeras: Cysteine-301 is important in TSH binding and receptor tertiary structure. Thyroid 1994;4:43-8.
293. Grasso YZ, Kim MR, Faiman C, Kohn LD, Tahara K and Gupta MK. Epitope heterogeneity of thyrotropin-blocking antibodies in Graves’ patients as detected with wild-type versus chimeric thyrotropin receptors. Thyroid 1999;9:521-37.
294. Kim WB, Chung HK, Lee HK, Kohn LD, Tahara K and Cho BY. Changes in epitopes for thyroid stimulation antibodies in Graves’ disease sera during treatment of hyperthyroidism: Therapeutic implications. J Clin Endocrinol Metab 1997;82:1953-9.
295. Shewring G and Smith BR. An improved radioreceptor assay for TSH receptor. Methods Enzymol 1982;17:409-17.
296. Costagliola S, Morganthaler NG, Hoermann R, Badenhoop K, Struck J, Freitag D, Poertl S, Weglohner W, Hollidt JM, Quadbeck B, Dumont JE, Schumm-Draeger PM, Bergmann A, Mann K, Vassart G and Usadel KH. Second generation assay for thyrotropin receptor antibodies has superior diagnostic sensitivity for Graves’ disease. J Clin Endocrinol Metab 1999;84:90-7.
297. Schott M, Feldkamp J, Bathan C, Fritzen R, Scherbaum WA and Seissler J. Detecting TSH-Receptor antibodies with the recombinant TBII assay: Technical and Clinical evaluation. 32 2000;:429-35.
298. Feldt-Rasmussen U. Analytical and clinical performance goals for testing autoantibodies to thyroperoxidase, thyroglobulin and thyrotropin receptor. Clin Chem 1996;42:160-3.
299. Giovanella L, Ceriani L and Garancini S. Clinical applications of the 2nd. generation assay for anti-TSH receptor antibodies in Graves’ disease. Evaluation in patients with negative 1st. generation test. Clin Chem Lab med 2001;39:25-8.
300. Momotani N, Noh JY, Ishikawa N and Ito K. Effects of propylthiouracil and methimazole on fetal thyroid status in mothers with Graves’ hyperthyroidism. J Clin Endocrinol Metab 1997;82:3633-6.
301. Brown RS, Bellisario RL, Botero D, Fournier L, Abrams CA, Cower ML et al. Incidence of transient congenital hypothyroidism due to maternal thyrotropin receptor-blocking antibodies in over one million babies. J Clin Endocrinol Metab 1996;81:1147-51.
302. Gerding MN, van der Meer Jolanda WC, Broenink M, Bakker O, W. WM and Prummel MF. Association of thyrotropin receptor antibodies with the clinical features of Graves’ opthalmopathy. Clin Endocrinol 2000;52:267-71.
303. Bartelena L, Marcocci C, Bogazzi F, Manetti L, Tanda ML, Dell’Unto E et al. Relation between therapy for hyperthyroidism and the course of Graves’ disease. N Engl J Med 1998;338:73-8.
304. Bech K. Immunological aspects of Graves’ disease and importance of thyroid stimulating immunoglobulins. Acta Endocrinol (Copenh) Suppl 1983;103:5-38.
305. Feldt-Rasmussen U. Serum thyroglobulin and thyroglobulin autoantibodies in thyroid diseases. Pathogenic and diagnostic aspects. Allergy 1983;38:369-87.
306. Nygaard B, Metcalfe RA, Phipps J, Weetman AP and Hegedus L. Graves’ disease and thyroid-associated opthalopathy triggered by 131I treatment of non-toxic goitre. J Endocrinol Invest 1999;22:481-5.
307. Ericsson UB, Tegler L, Lennquist S, Christensen SB, Stahl E and Thorell JI. Serum thyroglobulin in differentiated thyroid carcinoma. Acta Chir Scand 1984;150:367-75.
308. Haugen BR, Pacini F, Reiners C, Schlumberger M, Ladenson PW, Sherman SI, Cooper DS, Graham KE, Braverman LE, Skarulis MC, Davies TF, DeGroot LJ, Mazzaferri EL, Daniels GH, Ross DS, Luster M, Samuels MH, Becker DV, Maxon HR, Cavalieri RR, Spencer CA, McEllin K, Weintraub BD and Ridgway EC. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J Clin Endocrinol Metab 1999;84:3877-85.
309. Spencer CA, LoPresti JS, Fatemi S and Nicoloff JT. Detection of residual and recurrent differentiated thyroid carcinoma by serum Thyroglobulin measurement. Thyroid 1999;9:435-41.
310. Schlumberger M, C. P., Fragu P, Lumbroso J, Parmentier C and Tubiana M,. Circulating thyrotropin and thyroid hormones in patients with metastases of differentiated thyroid carcinoma: relationship to serum thyrotropin levels. J Clin Endocrinol Metab 1980;51:513-9.
311. Pacini F, Fugazzola L, Lippi F, Ceccarelli C, Centoni R, Miccoli P, Elisei R and Pinchera A. Detection of thyroglobulin in fine needle aspirates of nonthyroidal neck masses: a clue to the diagnosis of metastatic differentiated thyroid cancer. J Clin Endocrinol Metab 1992;74:1401-4.
312. Spencer CA, Takeuchi M and Kazarosyan M. Current Status and Performance Goals for Serum Thyroglobulin Assays. Clin Chem 1996;42:164-73.
313. Feldt-Rasmussen U and Schlumberger M. European interlaboratory comparison of serum thyroglobulin measurement. J Endocrinol Invest 1988;11:175-81.
314. Feldt-Rasmussen U, Profilis C, Colinet E, Black E, Bornet H, Bourdoux P et al. Human thyroglobulin reference material (CRM 457) 2nd part: Physicochemical characterization and certification. Ann Biol Clin 1996;54:343-348.
315. Schlumberger M J. Papillary and Follicular Thyroid Carcinoma. NEJM 1998;338:297-306.
316. Hjiyiannakis P, Mundy J and Harmer C. Thyroglobulin antibodies in differentiated thyroid cancer. Clin Oncol 1999;11:240-4.
317. Spencer CA. Recoveries cannot be used to authenticate thyroglobulin (Tg) measurements when sera contain Tg autoantibodies. Clin Chem 1996;42:661-3.
318. Massart C and Maugendre D. Importance of the detection method for thyroglobulin antibodies for the validity of thyroglobulin measurements in sera from patients with Graves’ disease. Clin Chem 2002;48:102-7.
319. Mariotti S, Barbesino G, Caturegli P, Marino M, Manetti L, Pacini F, Centoni R and Pinchera A. Assay of thyroglobulin in serum with thyroglobulin autoantibodies: an unobtainable goal? J Clin Endocrinol Metab 1995;80:468-72.
320. Black EG and Hoffenberg R. Should one measure serum thyroglobulin in the presence of anti-thyroglobulin antibodies? Clin Endocrinol 1983;19:597-601.
321. Schneider AB and Pervos R. Radioimmunoassay of human thyroglobulin: effect of antithyroglobulin autoantibodies. J Clin Endocrinol Metab 1978;47:126-37.
322. Spencer CA, Platler BW and Nicoloff JT. The effect of 125-I thyroglobulin tracer heterogeneity on serum Tg RIA measurement. Clin Chem Acta 1985;153:105-115.
323. Bugalho MJ, Domingues RS, Pinto AC, Garrao A, Catarino AL, Ferreira T, Limbert E and Sobrinho L. Detection of thyroglobulin mRNA transcripts in peripheral blood of individuals with and without thyroid glands: evidence for thyroglobulin expression by blood cells. Eur J Endocrinol 2001;145:409-13.
324. Bellantone R, Lombardi CP, Bossola M, Ferrante A,Princi P, Boscherini M et al. Validity of thyroglobulin mRNA assay in peripheral blood of postoperative thyroid carcinoma patients in predicting tumor recurrence varies according to the histologic type: results of a prospective study. Cancer 2001;92:2273-9.
325. Bojunga J, Roddiger S, Stanisch M, Kusterer K, Kurek R, Renneberg H, Adams S, Lindhorst E, Usadel KH and Schumm-Draeger PM. Molecular detection of thyroglobulin mRNA transcripts in peripheral blood of patients with thyroid disease by RT-PCR. Br J Cancer 2000;82:1650-5.
326. Smith B, Selby P, Southgate J, Pittman K, Bradley C and Blair GE. Detection of melanoma cells in peripheral blood by means of reverse transcriptase and polymerase chain reaction. Lancet 1991;338:1227-9.
327. Luppi M, Morselli M, Bandieri E, Federico M, Marasca R, Barozzi P, Ferrari MG, Savarino M, Frassoldati A and Torelli G. Sensitive detection of circulating breast cancer cells by reverse-transcriptase polymerase chain reaction of maspin gene. Ann Oncol 1996;7:619-24.
328. Ghossein RA and Bhattacharya S. Molecular detection and characterisation of circulating tumour cells and micrometastases in solid tumours. Eur J Cancer 2000;36:1681-94.
329. Ditkoff BA, Marvin MR, Yemul S, Shi YJ, Chabot J, Feind C et al. Detection of circulating thyroid cells in peripheral blood. Surgery 1996;120:959-65.
330. Arturi F, Russo D, Giuffrida D et al. Early diagnosis by genetic analysis of differentiated thyroid cancer metastases in small lymph nodes. J Clin Endocrinol Metab 1997;82:1638-41.
331. Ringel MD, Balducci-Silano PL anderson JS, Spencer CA, Silverman J, Sparling YH, Francis GL, Burman KD, Wartofsky L, Ladenson PW, Levine MA and Tuttle RM. Quantitative reverse transcription-polymerase chain reaction of circulating thyroglobulin messenger ribonucleic acid for monitoring patients with thyroid carcinoma. J Clin Endocrinol Metab 1998;84:4037-42.
332. Biscolla RP, Cerutti JM and Maciel RM. Detection of recurrent thyroid cancer by sensitive nested reverse transcription-polymerase chain reaction of thyroglobulin and sodium/iodide symporter messenger ribonucleic acid transcripts in peripheral blood. J Clin Endocrinol Metab 2000;85:3623-7.
333. Takano T, Miyauchi A, Yoshida H, Hasegawa Y, Kuma K and Amino N. Quantitative measurement of thyroglobulin mRNA in peripheral blood of patients after total thyroidectomy. Br J Cancer 2001;85:102-6.
334. Chelly J, Concordet JP, Kaplan JC and Kahn A. Illegitimate transcription: transcription of any gene in any cell type. Proc Natl Acad Sci USA 1989;86:2617-21.
335. Premawardhana LDKE, Phillips DW, Prentice LM and Smith BR. Variability of serum thyroglobulin levels is determined by a major gene. Clin Endocrinol 1994;41:725-9.
336. Bertelsen JB and Hegedus L. Cigarette smoking and the thyroid. Thyroid 1994;4:327-31.
337. Knudsen N, Bulow I, Jorgensen T, Perrild H, Oversen L and Laurberg P. Serum Tg – a sensitive marker of thyroid abnormalities and iodine deficiency in epidemiological studies. J Clin Endocrinol Metab 2001;86:3599-603.
338. Van den Briel T, West CE, Hautvast JG, Vulsma T, de Vijlder JJ and Ategbo EA. Serum thyroglobulin and urinary iodine concentration are the most appropriate indicators of iodine status and thyroid function under conditions of increasing iodine supply in schoolchildren in Benin. J Nutr 2001;131:2701-6.
339. Gardner DF, Rothman J and Utiger RD. Serum thyroglobulin in normal subjects and patients with hyperthyroidism due to Graves’ disease: effects of T3, iodide, 131I and antithyroid drugs. Clin Endocrinol 1979;11:585-94.
340. Feldt-Rasmussen U, Petersen PH, Date J and Madsen CM. Serum thyroglobulin in patients undergoing subtotal thyroidectomy for toxic and nontoxic goiter. J Endocrinol Invest 1982;5:161-4.
341. Hocevar M, Auersperg M and Stanovnik L. The dynamics of serum thyroglobulin elimination from the body after thyroid surgery. 1997;23:208-10.
342. Cohen JH, Ingbar SH and Braverman LE. Thyrotoxicosis due to ingestion of excess thyroid hormone. Endocrine Rev 1989;10:113-24.
343. Mitchell ML and Hermos RJ. Measurement of thyroglobulin in newborn screening specimens from normal and hypothyroid infants. Clin Endocrinol 1995;42:523-7.
344. Smallridge RC, De Keyser FM, Van Herle AJ, Butkus NE and Wartofsky L. Thyroid iodine content and serum thyroglobulin: clues to the natural history of destruction-induced thyroiditis. J Clin Endocrinol Metab 1986;62:1213-9.
345. Pacini F, Molinaro E, Lippi F, Castagna MG, Agate L, Ceccarelli C, Taddei D, Elisei R, Capezzone M and Pinchera A. Prediction of disease status by recombinant human TSH-stimulated serum Tg in the postsurgical follow-up of differentiated thyroid carcinoma. J Clin Endocrinol Metab 2001;86:5686-90.
346. Cobin RH. 1992. Medullary carcinoma of the thyroid. In Malignant tumors of the thyroid: clinical concepts and controversies. S. D. Cobin RH, editor. Springer-Verlag,, New York. 112-41.
347. Dunn JT. When is a thyroid nodule a sporadic medullary carcinoma? J Clin Endocrinol Metab 1994;78:824-5.
348. Pacini F, Fontanelli M, Fugazzola L and et. al. Routine measurement of serum calcitonin in nodular thyroid diseases allows the preoperative diagnosis of unsuspected sporadic medullary thyroid carcinoma. J Clin Endocrinol Metab 1994;78:826-9.
349. Mulligan LM, Kwok JB, Healey CS, Elsdon MJ, Eng C, Gardner E et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 1993;363:458-60.
350. Hofstra RM, Landvaster RM, Ceccherini I et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature 1994;367:375-6.
351. Heyningen van V. One gene-four syndromes. Nature 1994;367:319-20.
352. Becker KL, Nylen ES, Cohen R and Snider RH. Calcitonin: structure, molecular biology and actions. In: J.P. Beleziakian, L.E. Raisz, G.A.Rodan eds. Principle of bone biology, Academic Press, San Diego 1996;:471-4.
353. Motte P, Vauzelle P, Gardet P, Ghillani P, Caillou B, Parmentier C et al. Construction and clinical validation of a sensitive and specific assay for mature calcitonin using monoclonal anti-peptide antibodies. Clin Chim Acta 1988;174:35-54.
354. Zink A, Blind E and Raue F. Determination of serum calcitonin by immunometric two-site assays in normal subjects and patients with medullary thyroid carcinoma. Eur J Clin Chem Biochem 1992;30:831-5.
355. Engelbach M, Gorges R, Forst T, Pfutzner A, Dawood R, Heerdt S, Kunt T, Bockisch A and Beyer J. Improved diagnostic methods in the follow-up of medullary thyroid carcinoma by highly specific calcitonin measurements. J Clin Endocrinol Metab 2000;85:1890-4.
356. Milhaud G, Tubiana M, Parmentier C and Coutris G. Epithelioma de la thyroide secretant de la thyrocalcitonine. C.R. Acad. Sci (serie D), Paris 1968;266:608-10.
357. Guilloteau D, Perdrisot D, Calmettes C and et. al. Diagnosis of medullary carcinoma of the thyroid by calcitonin assay using monoclonal antibodies. J Clin Endocrinol Metab 1990;71:1064-7.
358. Niccoli P, Wion-Barbot N, Caron P and et.al. Interest of routine measurement of serum calcitonin (CT): study in a large series of thyroidectomized patients. J Clin Endocrinol Metab 1997;82:338-41.
359. Wells SA, Baylin SB, Linehan W, Farrell RE, Cox EB, Cooper CW. Provocative agents and the diagnosis of medullary carcinoma of the thyroid gland. Ann Surg 1978;188:139-41.
360. Gagel RF. The abnormal pentagastrin test. Clin Endocrinol 1996;44:221-2.
361. Wion-Barbot N, Schuffenecker I, Niccoli P et al. Results of the calcitonin stimulation test in normal volunteers compared with genetically unaffected members of MEN 2A and familial medullary thyroid carcinoma families. Ann Endocrinol 1997;58:302-8.
362. Barbot N, Calmettes C, Schuffenecker I et al. Pentagastrin stimulation test and early diagnosis of medullary carcinoma using an immunoradiometric assay of calcitonin: comparison with genetic screening in hereditary medullary thyroid carcinoma. J Clin Endocrinol Metab 1994;78:114-20.
363. Erdogan MF, Gullu S, Baskal N, Uysal AR, Kamel N, Erdogan G. Omeprazole: calcitonin stimulation test for the diagnosis follow-up and family screening in medullary carcinoma of the thyroid gland. Ann Surg 1997;188:139-41.
364. Vieira AEF, Mello MP, Elias LLK et al. Molecular and biochemical screening for the diagnosis and management of medullary thyroid carcinoma in multiple endocrine neoplasia Type 2A. Horm Metab Res 2002;34:202-6.
365. Wells SA, Chi DD, toshima K, Dehner LP, Coffin cm, Dowton SB, Ivanovich JL, DeBenedetti MK, Dilley WG and Moley JF. Predictive DNA testing and prophylactic thyroidectomy in patients at risk for multiple endocrine neoplasia type 2A. Ann Surg 1994;220:237-50.
366. Telander RL and Moir CR. Medullary thyroid carcinoma in children. Semin Pediatr Surg 1994;3:188-93.
367. Niccoli-Sire P, Murat A, Baudin E, Henry JF, Proye C, Bigorgne JC et al. Early or prophylactic thyroidectomy in MEN2/FMTC gene carriers: results in 71 thyroidectomized patients. Eur J Endocrinol 1999;141:468-74.
368. Niccoli-Sire P, Murat A, Rohmer V, Franc S, Chabrier G, Baldet L, Maes B, Savagner F, Giraud S, Bezieau S, Kottler ML, Morange S and Conte-Devolx B. Familial medullary thyroid carcinoma (FMTC) with non-cysteine RET mutations: phenotype-genotype relationship in large series of patients. J Clin Endocrinol Metab 2001;86:3756-53.
369. Body JJ, Chanoine JP, Dumon JC and Delange F. Circulating calcitonin levels in healthy children and subjects with congenital hypothyroidism from birth to adolescence. J Clin Endocrinol Metab 1993;77:565-7.
370. Gharib H, Kao PC and Heath H. Determination of silica-purified plasma calcitonin for the detection and management of medullary thyroid carcinoma: comparison of two provocative tests. Mayo Clin Proc 1987;62:373-8.
371. Telander R, Zimmerman D, Sizemore GW, van Heerden JA and Grant CS. Medullary carcinoma in children. Results of early detection and surgery. Arch Surg 1989;124:841-3.
372. Calmettes C, Ponder BA, Fisher JA and Raue F. Early diagnosis of multiple endocrine neoplasia type 2 syndrome: consensus statement. European community concerted action: medullary thyroid carcinoma. Eur J Clin Invest 1992;22:755-60.
373. Modigliani E, Cohen R, Campos JM, Conte-Devolx B, Maes B, Boneu A et al. Prognostic factors for survival and biochemical cure in medullary thyroid carcinoma: results in 899 patients. Clin Endocrinol 1998;48:265-73.
374. Machens A, Gimm O, Ukkat J et al. Improved prediction of calcitonin normalization in medullary thyroid carcinoma patients by quantitative lymph node analysis. Cancer 2000;88:1909-15.
375. Fugazzola L, Pinchera A, Lucchetti F et al. Disappearence rate of serum calcitonin after total thyroidectomy for medullary thyroid carcinoma. Internat J Biolog Markers 1994;9:21-4.
376. Vierhapper H, Raber W, Bieglmayer C and et.al. Routine measurement of plasma calcitonin in nodular thyroid diseases. J Clin Endocrinol Metab 1997;82:1589-93.
377. Fereira-Valbuena H, Fernandez de Arguello E, Campos G, Ryder E and Avellaneda A. Serum concentration of calcium and calcitonin in hyperthyroidism caused by Graves’ disease. Invest Clin 1991;32:109-14.
378. Lips CJM, Hoppener JWM and Thijssen JHH. Medullary thyroid carcinoma: role of genetic testing and calcitonin measurement. Ann Clin Biochem 2001;38:168-79.
379. Niccoli P, Brunet Ph, Roubicek C, Roux F, Baudin E, Lejeune PJ et al. Abnormal calcitonin basal levels and pentagastrin response in patients with chronic renal failure on maintenance hemodialysis. Eur J Endocrinol 1995;132:75-81.
380. Snider RH, Nylen ES and Becker KL. Procalcitonin and its component peptides in systemic inflammation: immunochemical characterization. J Invest Med 1997;47:552-60.
381. Russwurn S, Wiederhold M, Oberhoffer M et al. Molecular aspects and natural source of Procalcitonin. Clin Chem Lab Med 1999;37:789-97.
382. Niccoli P, Conte-Devolx B, Lejeune PJ, Carayon P, Henry JF, Roux F et al. Hypercalcitoninemia in conditions other than medullary cancers of the thyroid. Ann Endocrinol 1996;57:15-21.
383. Baudin E, Bidart JM, Rougier P et al. Screening for multiple endocrine neoplasia type 1 and hormonal production in apparently sporadic neuroendocrine tumors. J Clin Endocrinol Metab 1999;84:69-75.
384. DeLellis RA. C-Cell hyperplasia. In: Rosai J., Carangiu M.L., DeLellis R.A. eds: Atlas of Tumor Pathology, 3rd. series, Fasc 5: tumors of the thyroid gland. Washington DC, Armed Forces Institute of Pathology. 1992;:247-58.
385. Guyetant S, Wion-Barbot N and Rousselet MC. C-cell hyperplasia associated with chronic lymphocytic thyroiditis: a retrospective study of 112 cases. Hum Pathol 1994;25:514-21.
386. Albores-Saavedra J, Monforte H, Nadji M and Morales AR. C-Cell hyperplasia in thyroid tissue adjacent to follicular cell tumor. Hum Pathol 1988;19:795-9.
387. Mulligan LM, Marsh DJ, Robinson BG, Schuffenecker I, Zedenius J, Lips CJ et al. Genotype-phenotype correlation in multiple endocrine neoplasia type 2: report of the international RET mutation consortium. J Intern Med 1995;238:243-6.
388. Eng C, Clayton D, Schuffenecker I, Lenoir G, Cote G, Gagel RF et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA 1996;276:1575-9.
389. Ito S, Iwashita T, Asai N, Murakami H, Iwata Y, Sobue G et al. Biological properties of RET with cysteine mutations correlate with multiple endocrine neoplasia type 2A, familial medullary thyroid carcinoma and Hirschsprung’s disease phenotype. Cancer Res 1997;57:2870-2.
390. Heshmati HM, Gharib H, Khosla S et al. Genetic testing in medullary thyroid carcinoma syndromes: mutation types and clinical significance. Mayo Clin Proc 1997;72:430-6.
391. Berndt I, Reuter M, Saller B et al. A new hot spot for mutations in the RET proto-oncogene causing familial medullary thyroid carcinoma and multiple endocrine neoplasia type 2A. J Clin Endocrinol Metab 1998;83:770-4.
392. Komminoth P, Roth J, Muletta-Feurer S, Saremaslani P, Seelentag WKF and Heitz PU. RET proto-oncogene point mutations in sporadic neuroendocrine tumors. J Clin Endocrinol Metab 1996;81:2041-6.
393. Conte-Devolx B, Schuffenecker I, Niccoli P, Maes B, Boneu A, Barbot N et al. Multiple Endocrine Neoplasia Type 2: Management of patients and subjects at risk. Horm Res 1997;47:221-6.
394. Smith DP, Houghton C and Ponder BA. Germline mutation of RET codon 883 in two cases of de novo MEN2B. Oncogene 1997;15:1213-7.
395. Carlson KM, Bracamontes J, Jackson CE, Clark R, Lacroix A, Wells SA Jr et al. Parent-of-origin effects in multiple endocrine neoplasia type 2B. J Hum Genet 1994;55:1076-82.
396. Moers AMJ, Landsvater RM, Schaap C, van Veen JM, de Valk IAJ, Blijham GH et al. Familial medullary thyroid carcinoma: not a distinct entity/ Genotype-phenotype correlation in a large family: familial medullary thyroid carcinoma revisited. Am J Med 1996;101:634-41.
397. Dunn JT. Iodine deficiency – the next target for elimination. N Engl J Med 1992;326:267-8.
398. Delange F. Correction of iodine deficiency: benefits and possible side effects. Eur J Endocrinol 1995;132:542-3.
399. Dunn JT. Whats happening to our iodine. J Clin Endocrinol Metab 1998;83:3398-3400.
400. Knudsen N, Christiansen E, Brandt-Christensen M, Nygaard B and Perrild H. Age- and sex-adjusted iodine/creatinine ratio. A new standard in epidemiological surveys? Evaluation of three different estimates of iodine excretion based on casual urine samples and comparison to 24 h values. Eur J Clin Nutr 2000;54:361-3.
401. Aumont G and Tressol JC. Improved routine method for the determination of total iodine in urine and milk. Analyst 1986;111:841-3.
402. Unak P, Darcan S, Yurt F, Biber Z and Coker M. Determination of iodine amounts in urine and water by isotope dilution analysis. Biol Trace Elem Res Winter 1999;71-2:463-70.
403. Kilbane MT, Ajja RA, Weetman AP, Dwyer R, McDermott EWM, O’Higfins NJ and Smyth PPA. Tissue Iodine content and serum mediated 125I uptake blocking activityin breast cancer. J Clin Endocrinol Metab 2000;85:1245-50.
404. Liberman CS, Pino SC, Fang SL, Braverman LE and Emerson CH. Circulating iodine concentrations during and after pregnancy. J Clin Endocrinol Metab 1998;83:3545-9.
405. Vought RL, London WT, Lutwak L and Dublin TD. Reliability of estimates of serum inorganic iodine and daily faecal and urinary iodine excretion from single casual specimens. J Clin Endcorinol Metab 1963;23:1218-28.
406. Smyth PPA, Darke C, Parkes AB, Smith DF, Hetherton AM and Lazarus JH. Assessment of goitre in an area of endemic iodine deficiency. Thyroid 1999;9:895-901.
407. Thomson CD, Smith TE, Butler KA and Packer MA. An evaluation of urinary measures of iodine and selenium status. J Trace Elem Med and Biol 1996;10:214-22.
408. Als C, Helbling A, Peter K, Haldimann M, Zimmerli B and Gerber H. Urinary iodine concentration follows a circadian rhythm: A study with 3023 spot urine samples in adults and children. J Clin Endocrinol Metab 2000;85:1367-9.
409. Lightowler H and Davis JG. Iodine intake and iodine deficiency in vegans as assessed by the duplicate-portion technique and urinary iodine excretion. Br. J Nutr 1999;80:529-35.
410. Utiger RD. Maternal hypothyroidism and fetal development. N Engl J Med 1999;341:601-2.
411. Aboul-Khair S, Crooks J, Turnbull AC and Hytten FE. The physiological changes in thyroid function during pregnancy. Clin Sci 1964;27:195-207.
412. Smyth PPA, Smith DF, Radcliff M and O’Herlihy C. Maternal iodine status and thyroid volume during pregnancy: correlation with neonatal intake. J Clin Endocrinol Metab 1997;82:2840-3.
413. Gunton JE, Hams GH, Fiegert M and McElduff A. iodine deficiency in ambulatory participants at a Sydney teaching hospital: Is Australia truly iodine replete? Med J Aust 1999;171:467-70.
414. Smyth PPA. Variation in iodine handling during normal pregnancy. Thyroid 1999;9:637-42.
415. Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc. National Academic Press 2001
416. Koutras DA, Papadoupoulos SN, Sfontouris JG and Rigopoulos GA. Comparison of methods for measuring the plasma inorganic iodine and the absolute iodine uptake by the thyroid gland. J Clin Endocrinol Metab 1968;28:757-60.
417. Mizukami Y, Michigishi T, Nonomura A, Hashimoto T, Tonami N, Matsubara F et al. Iodine-induced hypothyroidism: a clinical and histological study of 28 patients. J Clin Endocrinol Metab 1993;76:466-71.
418. Heymann WR. Potassium iodide and the Wolff-Chaikhoff effect: relevance for the dermatologist. J Am Acad Dermatol 2000;42:490-2.s
419. Stanbury JB, Ermans AE, Bourdoux P, Todd C, Oken E, Tonglet R, Bidor G, Braverman LE and Medeiros-Neto G. Iodine-induced hyperthyroidism: occurrence and epidemiology. Thyroid 1998;8:83-100.
420. Roti E and Uberti ED. Iodine excess and hyperthyroidism. Thyroid 2001;5:493-500.
421. Baltisberger BL, Minder CE and Burgi H. Decrease of incidence of toxic nodular goitre in a region of Switzerland after full correction of mild iodine deficiency. Eur J Endocrinol 1995;132:546-9.
422. Bacher-Stier RG, Totsch M, Kemmler G, Oberaigner W and Moncayo R. Incidence and clinical characteristics of thyroid carcinoma after iodine prophylaxis in an endemic goiter country. Thyroid 1997;7:733-41.
423. Barakat MCD, Carson D, Hetherton AM, Smyth PPA and Leslie H. Hypothyroidism secondary to topical iodine treatment in infants with spina bifida. Acta Paediat 1994;83:741-3.
424. Martino E, Safran M, Aghino-Lombardi F, Rajatanavin R, Lenziardi M, Fay M et al. Environmental iodine intake and thyroid dysfunction during chronic amiodarone therapy. Ann Intern Med 1984;101:28-34.
425. Rose NR, Rasooly L, Saboori AM and Burek CL. Linking iodine with autoimmune thyroiditis. Environmental Health Perspectives 1999;107:749-52.
426. Premawardhana LDKEPA, Smyth PPA, Wijeyaratne C, Jayasinghe A, De Silva H and Lazarus JH. Increased prevalence of thyroglobulin antibodies in Sri Lankan schoolgirls – is iodine the cause? Eur J Endocrinol 2000;143:185-8.
427. Costa A, Testori OB, Cenderelli C, Giribone G and Migliardi M. Iodine content of human tissues after administration of iodine containing drugs or contrast media. J Endocrinol Invest 1978;1:221-5.
428. May W, Wu D, Eastman C, Bourdoux P and Maberly G. Evaluation of automated urinary iodine methods: problems of interfering substances identified. Clin Chem 1990;35:865-9.
429. Lauber K. Iodine determination in biological material. Kinetic measurement of the catalytic activity of iodine. Analyt Chem 1975;47:769-71.
430. Mantel M. Improved method for the determination of iodine in urine. Clin Chim Acta 1971;33:39-44.
431. Dunn JT, Crutchfield HE, Gutenkunst R and Dunn AD. Two simple methods for measuring iodine in urine. Thyroid 1993;3:119-23.
432. May SL, May WA, Bourdoux PP, Pino S, Sullivan KM and Maberly GF. Validation of a simple, manual urinary iodine method for estimating the prevalence of iodine-deficiency disorders and interlaboratory comparison with other methods. J Clin Nutr 1997;65:1441-5.
433. Ohashi T, Yamaki M, Pandav SC, Karmarkar GM and Irie M. Simple microplate method for determination of urinary iodine. Clin Chem 2000;46:529-36.
434. Rendl J, Seybold S and Borner W. Urinary iodine determined by paired-ion reverse-phase HPLC with electrochemical detection. Clin Chem 1994;40:908-13.
435. Tsuda K, Namba H, Nomura T, Yokoyama N, Yamashita S, Izumi M and Nagataki S. Automated Measurement of urinary iodine with use of ultraviolet radiation. Clin Chem 1995;41:581-5.
436. Haldimann M, Zimmerli B, Als C and Gerber H. Direct determination of urinary iodine by inductively coupled plasma mass spectrometry using isotope dilution with iodine-129. Clin Chem 1998;44:817-24.
437. Mura P, Piriou A, Guillard O, Sudre Y and Reiss D. Dosage des iodures urinares par electrode specifique: son interet au cours des dysthyroides. Ann Biol Clin 1985;44:123-6.
438. Allain P, Berre S, Krari N, Laine-Cessac P, Le Bouil A, Barbot N, Rohmer V and Bigorgne JC. Use of plasma iodine assays for diagnosing thyroid disorders. J Clin Pathol 1993;46:453-5.
439. Vander JB, Gaston EA and Dawber TR. The significance of nontoxic thyroid nodules: Final report of a 15-year study of the incidence of thyroid malignancy. Ann Intern Med 1968;69:537-40.
440. Rojeski MT and Gharib H. Nodular thyroid disease: Evaluation and management. N Engl J Med 1985;313:428-36.
441. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med 1993;328:553-9.
442. Kirkland RT and Kirkland JL. Solitary thyroid nodules in 30 children and report of a child with thyroid abscess. Pediatrics 1973;51:85-90.
443. Rallison ML, Dobyns EM, Keating FR, Rall J and Tyler E. Thyroid nodularity in children. JAMA 1975;233:1069-72.
444. Khurana KK, Labrador E, Izquierdo R, Mesonero CE and Pisharodi LR. The role of fine-needle aspiration biopsy in the management of thyroid nodules in children, adolescents and young adults: A multi-institutional study. Thyroid 1999;4:383-6.
445. Aghini-Lombardi F, Antonangeli L, Martino E, Vitti P, Maccherini D, Leoli F, Rago T, Grasso L, Valeriano R, Balestrieri A and Pinchera A. The spectrum of thyroid disorders in an iodine-deficient community: the Pescopanano Survey. J Clin Endocrinol Metab 1999;84:561-6.
446. Hamburger JI, Husain M, Nishiyama R, Nunez C and Solomon D. Increasing the accuracy of fine-needle biopsy for thyroid nodules. Arch Pathol Lab Med 1989;113:1035-41.
447. Hundahl SA, Cady B, Cunningham MP, Mazzaferri E, McKee RF, Rosai J, Shah JP, Fremgen AM, Stewart AK and Holzer S. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the United States during 1996. Cancer (Cytopathol) 2000;89:202-17.
448. Leenhardt L, Hejblum G, Franc B, Du Pasqueir Fediaevsky L, Delbot T, De Guillouzic D, Menegaux F, Guillausseau C, Hoang C, Turpin G and Aurengo A. Indications and limits of ultrasound-guided cytology in the management of nonpalpable thyroid nodules. J Clin Endocrinol Metab 1999;84:24-8.
449. Braga M, Cavalcanti TC, Collaco LM and Graf H. Efficacy of ultrasound-guided fine-needle aspiration biopsy in the diagnosis of complex thyroid nodules. J Clin Endocrinol Metab 2001;86:4089-91.
450. Cochand-Priollet B, Guillausseau P, Chagnon S, Hoang C, Guillausseau-Scholer C, Chanson P, Dahan H, Warnet A, Tran Ba Huy PT and Valleur P. The diagnostic value of fine-needle aspiration biopsy under ultrasonoraphy in nonfunctional thyroid nodules: a prospective study comparing cytologic and histologic findings. Am J Med 1994;97:152-7.
451. Takashima S, Fukuda H and Kobayashi T. Thyroid nodules: Clinical effect of ultrasound-guided fine needle aspiration biopsy. J Clin Ultrasound 1994;22:535-42.
452. Gharib H. Fine-needle aspiration biopsy of thyroid nodules: Advantages, limitations and effect. Mayo Clin Proc 1994;69:44-9.
453. Hamberger B, Gharib H, Melton LF III, Goellner JR and Zinsmeister AR. Fine-needle aspiration biopsy of thyroid nodules. Impact on thyroid practice and cost of care. Am J Med 1982;73:381-4.
454. Grant CS, Hay ID, Gough IR, McCarthy PM and Goelliner JR. Long-term follow-up of patients with benign thyroid fine-needle aspiration cytologic diagnoses. Surgery 1989;106:980-6.
455. Liel Y, Ariad S and Barchana M. Long-term follow-up of patients with initially benign fine-needle aspirations. Thyroid 2001;11:775-8.
456. Belfiore A, La Rosa G, La Porta GA, Giuffrida D, Milazzo G, Lupo L, Regalbuto C and V. R. Cancer Risk in patients with cold thyroid nodules: Relevance of iodine intake, sex, age and multinodularity. J Amer Med 1992;93:363-9.
457. Tuttle RM, Lemar H and Burch HB. Clinical features associated with an increased risk of thyroid malignancy in patients with follicular neoplasia by fine-needle aspiration. Thyroid 1998;8:377-83.
458. Kumar H, Daykin J, Holder R, Watkinson JC, Sheppard M and Franklyn JA. Gender, clinical findings and serum thyrotropin measurements in the prediction of thyroid neoplasia in 1005 patients presenting with thyroid enlargement and investigated by fine-needle aspiration cytology. Thyroid 1999;11:1105-9.
459. Moosa M and Mazzaferri EL. Outcome of differentiated thyroid cancer diagnosed in pregnant women. J Clin Endocrinol Metab 1997;82:2862-6.
460. Oertel YC. A pathologist trying to help endocrinologists to interpret cytology reports from thyroid aspirates. J Clin Endocrinol Metab 2002;87:1459-61.
461. De Micco, Zoro P, Garcia S, Skoog L, Tani EM, C. PK and Henry JF. Thyroid peroxidase immunodetection as a tool to assist diagnosis of thyroid nodules on fine-needle
aspiration biopsy. Eur J Endocrinol 1994;131:474-9.
462. Faroux MJ, Theobald S, Pluot M, Patey M and Menzies D. Evaluation of the monoclonal antithyroperoxidase MoAb47 in the diagnostic decision of cold thyroid nodules by fine-needle aspiration. Pathol Res Pract 1997;193:705-12.
463. Inohara H, Honjo Y, Yoshii T, Akahani S, Yoshida J, Hattori K, Okamoto S, Sawada T, Raz A and Kubo T. Expression of galectin-3 in fine-needle aspirates as a diagnostic marker differentiating benign from malignant thyroid neoplasms. Cancer 1999;85:2475-84
464. Medeiros-Neto G, Nascimento MC, Bisi H, Alves VA, Longatto-Filho A and Kanamura CT. Differential reactivity for Galectin-3 in Hurthle Cell Adenomas and Carcinomas. Endocr Pathol 2001;12:275-9.
465. Saggiorato E, Cappia S, De Guili P, Mussa A, Pancani G, Caraci P, Angeli A and Orlandi F. Galectin -3 as a presurgical immunocytodiagnostic marker of minimally invasive follicular carcinoma. J Clin Endocrinol Metabl 2001;86:5152-8.
466. Bartolazzi A, Gasbarri A, Papotti M, Bussolati G, Lucante T, Khan A, Inohara H, Marandino F, Orkandi F, Nardi F, Vacchione A, Tecce R and Larsson O. Application of an immunodiagnostic method for improving preoperative diagnosis of nodular thyroid lesions. Lancet 2001;357:1644-50.
467. Goellner JR. Problems and pitfalls in thyroid cytology. Monogr Pathol 1997;39:75-93.
468. Oertel YC, O. J. Diagnosis of benign thyroid lesions: fine-needle aspiration and histopathologic correlation. Ann Diagn Pathol 1998;2:250-63.
469. Baldet L, Manderscheid JC, Glinoer D, Jaffiol C, Coste-Seignovert B and Percheron C. The management of differentiated thyroid cancer in Europe in 1988. Results of an international survey. Acta Endocrinol (Copenh) 1989;120:547-58.
470. Baloch ZW, Fleisher S, LiVolsi VA and Gupta PK. Diagnosis of “follicular neoplasm”: a gray zone in thyroid fine-needle aspiration cytology. Diagn Cytopathol 2002;26:41-4.
471. Herrmann ME, LiVolsi VA, Pasha TL, Roberts SA, Wojcik EM and Baloch ZW. Immunohistochemical expression of Galectin-3 in benign and malignant thyroid lesions. Arch Pathol Lab Med 2002;126:710-13.
472. Leteurtre E, Leroy Z, Pattou F, Wacrenier A, Carnaille B, Proye C and Lecomte-Houcke M. Why do frozen sections have limited value in encapsulated or minimally invasive follicular carcinoma of the thyroid? Amer J Clin Path 2001;115:370-4.
473. Stojadinovic A, Ghossein RA, Hoos A, Urist MJ, Spiro RH, Shah JP, Brennan MF, Shaha AR and Singh B. Hurthle cell carcinoma: a critical histopathologic appraisal. J Clin Oncol 2001;19:2616-25.
474. Carmeci C, Jeffrey RB, McDougall IR, Nowels KW and Weigel RJ. Ultrasound-guided fine-needle aspiration biopsy of thyroid masses. Thyroid 1998;8:283-9.
475. Yang GCH, Liebeskind D and Messina AV. Ultrasound-guided fine-needle aspiration of the thyroid assessed by ultrafast Papanicoulaou stain: Data from 1135 biopsies with a two- six-year follow-up. Thyroid 2001;6:581-9.
476. Fisher DA, Dussault JH, Foley TP, Klein AH, LaFranchi S, Larsen PR, Mitchell NL, Murphey WH and Walfish PG. Screening for congenital hypothyroidism: results of screening one million North American infants. J Pediatr 1979;94:700.
477. Brown AL, Fernhoff PM, Milner J, McEwen C and Elsas LS. Racial differences in the incidence of congenital hypothyroidism. J Pediatr 1981;99:934-.
478. LaFranchi SH, Dussault JH, Fisher DA, Foley TP and Mitchell ML. Newborn screening for congenital hypothyroidism: Recommended guidelines. Pediatrics 1993;91:1203-9.
479. Gruters A, Delange F, Giovanelli G, Klett M, Richiccioli P, Torresani T et al. Guidelines for neonatal screening programmes for congenital hypothyroidism. Pediatr 1993;152:974-5.
480. Toublanc JE. Guidelines for neonatal screening programs for congenital hypothyroidism. Acta Paediatr 1999;88 Suppl 432:13-4.
481. Vulsma T, Gons MH and de Vijlder JJ. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N Engl J Med 1989;321:13-6.
482. Gruneiro-Papendieck L, Prieto L, Chiesa A, Bengolea S, Bossi G and Bergada C. Usefulness of thyroxine and free thyroxine filter paper measurements in neonatal screening for congenital hypothyroidism of preterm babies. J Med Screen 2000;7:78-81.
483. Hanna DE, Krainz PL, Skeels MR, Miyahira RS, Sesser DE and LaFranchi SH. Detection of congenital hypopituitary hypothyroidism: Ten year experience in the Northwest Regional Screening Program. J Pediatr 1986;109:959-64.
484. Fisher DA. Hypothyroxinemia in premature infants: is thyroxine treatment necessary? Thyroid 1999;9:715-20.
485. Wang ST, Pizzalato S and Demshar HP. Diagnostic effectiveness of TSH screening and of T4 with secondary TSH screening for newborn congenital hypothyroidism. Clin Chim Acta 1998;274:151-8.
486. Delange F. Screening for congenital hypothyroidism used as an indicator of the degree of IDD and its control. Thyroid 1998;8:1185-92.
487. Law WY, Bradley DM, Lazarus JH, John R and Gregory JW. Congenital hypothyroidism in Wales (1982-93): demographic features, clinical presentation and effects on early neurodevelopment. Clin Endocrinol 1998;48:201-7.
488. Mei JV, Alexander JR, Adam BW and Hannon WH. Use of filter paper for the collection and analysis of human whole blood specimens. J Nutr 2001;131:1631S-6S.
489. LaFranchi SH, Hanna CE, Krainz PL, Skeels MR, Miyahira RS and Sesser DE. Screening for congenital hypothyroidism with specimen collection at two time periods: Results of the Northwest Regional Screening Program. J Pediatr 1985;76:734-40.
490. Zakarija M, McKenzie JM and Eidson MS. Transient neonatal hypothyroidism: Characterization of maternal antibodies to the Thyrotropin Receptor. J Clin Endocrinol Metab 1990;70:1239-46.
491. Matsuura N, Yamada Y, Nohara Y, Konishi J, Kasagi K, Endo K, Kojima H and Wataya K. Familial neonatal transient hypothyroidism due to maternal TSH-binding inhibitor immunoglobulins. N Engl J Med 1980;303:738-41.
492. McKenzie JM and Zakaria M. Fetal and neonatal hyperthyroidism and hypothyroidism due to maternal TSH receptor antibodies. Thyroid 1992;2:155-9.
493. Vogiatzi MG and Kirkland JL. Frequency and necessity of thyroid function tests in neonates and infants with congenital hypothyroidism. Pediatr 1997;100.
494. Pohlenz J, Rosenthal IM, Weiss RE, Jhiang SM, Burant C and Refetoff S. Congenital hypothyroidism due to mutations in the sodium/iodide symporter. Identification of a nonsense mutation producing a downstream cryptic 3′ splice site. J Clin Invest 1998;101:1028-35.
495. Nordyke RA, Reppun TS, Mandanay LD, Wood JC, Goldstein AP and Miyamoto LA. Alternative sequences of thyrotropin and free thyroxine assays for routine thyroid function testing. Quality and cost. Arch Intern Med 1998;158:266-72.
496. Hansen D, Bennedbaek FN, Hoier- Madsen M, Jacobsen BB, and Hegedus L. Thyroid function, morphology and autoimmunity in patients with insulin-dependent diabetes mellitus. Eur J Endocrinol 1999;140:512-8.
497. Pedersen OM, Aardal NP, Larssen TB, Varhaug JE, Myking O, and Vik-Mo H. The value of ultrasonography in predicting autoimmune thyroid disease. Thyroid 2000;10:251-9.
498. Harach HR, Solís Sánchez S, Williams ED: Pathology of the autonomously functioning (hot) thyroid nodule. Ann Diagn Pathol 2002;6:10-19.
499. Pretell EA, Delange F, Hostalek U, Corigliano S, Barreda L, Higa AM, Altschuler N,Barragán, D, Cevallos JL, Gonzales O, Jara JA, Medeiros-Neto G, Montes JA, Muzzo S, Pacheco VM and Cordero L. Iodine nutrition improves in Latin America. Thyroid 2004;14:590-9.







